Columbus City Schools
ABC: Acid Base Chemistry
Bubble, bubble, boil and trouble! What causes common substances like baking soda and vinegar to react the way they do? Welcome your junior chemists to the wonders of acid-base chemistry using a comprehensive and fun...
Virginia Department of Education
A Crystal Lab
Young chemists grow ionic crystals, metallic crystals, and supersaturated crystals in three different lab experiments. Observing these under a microscope allows pupils to compare the various structures.
Kenan Fellows
Unit 2: Chemistry Review
What exactly goes into the medications people take every day? Scholars learn about the chemistry of medications in the second of a four-part series on Pharmacology. Over the course of two weeks, class members complete seven experiments,...
Royal Society of Chemistry
Apparatus Diagrams 2
Ready to heat things up in the lab, but your class doesn't know its way around a Bunsen burner? Scholars get familiar with heating apparatuses through a series of puzzles designed to promote vocabulary and reasoning skills. The...
SRI International
Science of Water
Water is crucial to survival. Scholars gain an appreciation for water by reading about it, learning about its atomic properties, and investigating its properties through six stations in a lab activity.
Museum of Science
Gum Chemistry
Gum be gone! Scholars conduct an experiment to find the best substance that would help remove gum from the bottom of a shoe. They test peanut butter, petroleum jelly, olive oil, vinegar, and rubbing alcohol in their experiments.
Howard Hughes Medical Institute
Got Lactase? The Co-Evolution of Genes and Culture
Does the human body evolve as quickly as human culture? With a stellar 15-minute video, explore the trait of lactose intolerance. Only about 1/3 of human adults seem to still have the enzyme lactase and therefore, the ability to digest...
Kenan Fellows
Unit 4: The Brain
Drugs interact with the brain to alter moods, emotions, and behaviors by changing the brain's chemistry, perceptions, and interactions. The final lesson in the Pharmacology unit shows scholars experiments, has them complete four labs,...
Kenan Fellows
Unit 3: How Drugs Enter/Exit the Body
The third of a four-part series on Pharmacology teaches scholars how drugs enter and exit the body, how they act inside the body, how they affect the brain, and more. Over the course of the unit, groups complete two labs and one...
Normal Community High School
Mole Calculations
You didn't know you'd find moles in chemistry class! Through the introduction of moles and molar volume at STP, classes see how to calculate moles using a given chemical equation. The presentation includes a short review of ions in...
Next Generation Science Storylines
Why Do Some Things Get Colder (or Hotter) When They React?
Some reactions absorb heat while others release it. Young scholars investigate both types of reactions in a 12-lesson unit. Each lesson presents a lab investigation that monitors temperature and considers the types of reactions taking...
Virginia Department of Education
Acid-Base Theory
Litmus paper, why so blue? A chemistry instructional activity includes a pre-lab activity, practice calculating pH, an experiment measuring the pH in acids and bases, a titration demonstration, and a titration experiment.
Consortium for Ocean Science Exploration and Engagement (COSEE)
Carbon Dioxide & Krill: Impacts
What effects do temperature and carbon dioxide levels have on the zooplankton of Antarctica? This concluding lesson plan in a short unit on climate change and the ocean helps environmental scientists answer these questions. After...
Science Geek
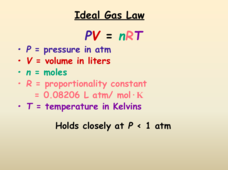
The Ideal Gas Law
When doing a gas lab, you might feel under pressure. A short presentation discusses the Ideal Gas Law. It begins with the units for each variable, then describes the behavior of real gases. The lesson concludes with a comparison of...
American Chemical Society
Combustion and Burning
On Earth, a candle flame points up, but on the International Space Station, it forms a sphere. Young scientists practice their skills by recording observations before, during, and after a candle burns. Chemical and physical...
Kenan Fellows
Reaction Stoichiometry—How Can We Make Chalk?
What is a reasonable percent yield in the manufacturing process? Scholars develop a process for producing chalk in the third lesson of a six-part series. Then, they must determine the theoretical and percent yield. Discussions about...
Science Geek
Thermochemical Calculations
Viewers learn where the heat goes when phase changes take place with a presentation that explains the latent heat of phase changes, or, more specifically, the molar heat of fusion, solidification, vaporization, and condensation. The show...
SRI International
Nanofiltration
How can everyone in the world have access to clean drinking water? Throughout the lesson, learners read about and listen to how water is filtered, what the filtration process removes, and the best ways to filter. They explore the...
Kenan Fellows
Qualitative Kinetics: Examining the Effect of an Enzyme on a Reaction
Scholars learn about kinetics and buffers as they use qualitative and quantitative methods to understand enzyme rates and buffer capacity. The application of Beer's Law and spectrophotometry solidifies pupils' knowledge in the first of...
Normal Community High School
Density
Change the density of water by adding minerals. The presentation discusses density—from the definition to calculations—and applies it to the real world. It briefly mentions specific gravity, and finishes by showing Archimedes'...
Teach Engineering
Density and Miscibility
The liquids did not mix — so what do density columns have to do with it? The seventh part in a series of nine provides the theoretical explanation of why density columns do not mix. The lesson covers the topics related to...
NOAA
Ocean Layers I
How is it possible for ocean water to have layers? The sixth installment of a 23-part NOAA Enrichment in Marine sciences and Oceanography (NEMO) program investigates factors that cause different water densities to occur. Experiments...
National Institute of Open Schooling
Atomic Structure
Learners explain historical findings such as Rutherford and Bohr's contributions, explain wave particle duality, and formulate Heinsenberg's uncertainty principle. They also draw s, p, and d orbitals, explain more historical findings,...
Science Geek
Ionic Compound Formulas
By contrasting cations and anions, this presentation shows how to predict ionic charges by periodic groups. The slides conclude with a few guided practice problems for writing ionic compound formulas.
Other popular searches
- Acid Base Chemistry Labs
- Chemistry Labs of Hydrates
- High School Chemistry Labs
- Christmas Chemistry Labs
- Thermo Chemistry Labs
- Holiday Chemistry Labs
- Introduction Chemistry Labs
- Therm Chemistry Labs
- Online Chemistry Labs
- Chemistry Labs With Candy