Royal Society of Chemistry
Organic Molecules Day—Chemistry Outreach
In search of an organic lab that employs real-life techniques and analysis methods? Groups carry out the nitration of methyl benzoate, then attempt to determine the number and location of the nitro groups added to the benzene ring....
Royal Society of Chemistry
Green Plastics—Chemistry Outreach
How do green plastics reduce waste and environmental pollution? Budding scientists create and test a variety of compounds used in green plastics during an insightful experiment. Beginning with startling statistics and ending with...
Royal Society of Chemistry
Electrochromic Polymer—Chemistry Outreach
From windows that tint themselves to OLED technology, electrochromic polymers are redefining our ideas about conducting materials! Introduce your chemistry class to the emerging trend with an exciting lab activity. Budding materials...
Royal Society of Chemistry
A Cartesian Diver—Classic Chemistry Experiments
Sometimes the simplest experiments leave the biggest impression! Introduce young chemists to the Cartesian Diver by having them make one of their own. Use the Diver to further their study of liquids and gases, as well as compression.
Royal Society of Chemistry
The Treatment of Oil Spills—Microscale Chemistry
When oil spills happen, how is the oil cleaned up? Pupils of polymer science discover an amazing substance that turns oil into a solid during a microscale experiment. Individuals observe oil or paraffin before and after addition of the...
Royal Society of Chemistry
Polymers—Gifted and Talented Chemistry
Polymers are an important part of our day-to-day lives, but how much do your pupils know about them? Learn the basics and beyond in a series of activities designed to build skills in observation, planning, organic chemistry, and bonding.
Royal Society of Chemistry
Aspirin—The Wonder of Medicine
What do aspirin and the willow tree have in common? Scholars of chemical synthesis engage in a fascinating reaction to make their own aspirin samples. The lab uses thin layer chromatography analysis, includes stoichiometric calculations,...
Royal Society of Chemistry
Investigating Temperature Changes on Evaporating Liquids—Microscale Chemistry
Is there more to evaporation than just less liquid? Show young scientists the energy transformation that occurs during a phase change through a series of simple experiments. Lab partners place drops of water, ethanol, and ethoxyethane on...
Cornell University
Polymerization
Explore condensation polymerization and additive polymerization through hands-on activities. Young scholars first model additive polymerization with paperclips. They finish the activity by using condensation polymerization to create a...
Cornell University
Splitting Water with Electricity
Explore how electricity splits water molecules into hydrogen and oxygen. Learners begin by calculating the voltage necessary to separate the water. They then perform the experiment and measure the ratio of hydrogen and oxygen bubbles.
Concord Consortium
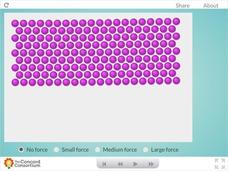
Plastic Forces
Plastic is fantastic! But, why does it behave the way it does? Science sleuths investigate the behavior of plastic in response to applied forces using an interactive. The resource allows users to bend a sample of plastic using three...
Concord Consortium
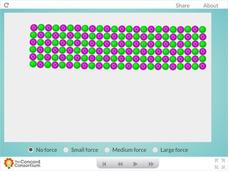
Metal Forces
Are you all bent out of shape, trying to find a great resource that illustrates the properties of metals? Show science scholars the unique world of metallic bonding with a hands-on activity. Users apply three levels of force to a sample...
Concord Consortium
Ceramic Forces
Why are bricks more likely to break than bend? Young science scholars peer inside a ceramic block and examine the effects of downward force at the molecular level. Learners can apply three different levels of force before observing their...
Cornell University
Radical Reactions
The radical reactions of polymers seems abstract to many pupils, but this lesson turns them into a fun building game. Scholars use dice and building pieces to build polymers. Then, they determine the theoretical and experimental weight...
Concord Consortium
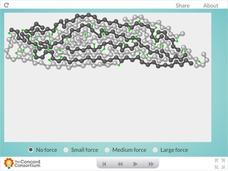
Tire Forces
No need to tread lightly on this piece of tire rubber! Polymer science pupils observe the behavior of rubber with an interesting interactive. Users apply three different levels of force to a sample, then watch how they affect the polymer...
Concord Consortium
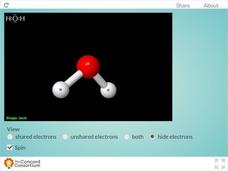
Unshared Electrons and the "Bent" Shape
Why is water always so bent out of shape? Scholars investigate the molecular geometry of the water molecule using a 3-D resource. The interactive features options such as rotation and the ability to view electron pairs.
Concord Consortium
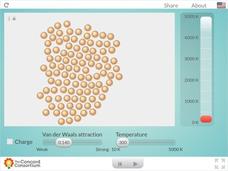
Charged and Neutral Atoms
Do charged and neutral particles behave differently as they undergo phase changes? Science sleuths examine two types of attractive forces using an informative interactive. Pupils can vary the amount of Van der Waals attraction present...
Concord Consortium
Factors Affecting London Dispersion Attractions
How can non-polar molecules be attracted to one another? Introduce the phenomenon of London dispersion forces to young chemists through an entertaining interactive. Pupils choose from a variety of molecular shape combinations, then pull...
Concord Consortium
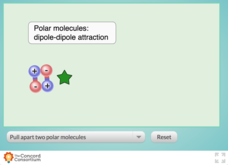
Polarity and Attractive Strength
Teaching intermolecular forces can be quite a stretch! Chemistry scholars experiment with the attractive strength between polar molecules using an interactive resource. Learners test molecules of low, medium, and high polarities by...
Concord Consortium
Oil and Water
If you don't get along with someone, it's said that the two of you are like oil and water. Why is this? Explore the phenomenon and explain the phrase in one resource! Science superstars first observe samples of oil and water together....
Concord Consortium
Comparing Dipole-Dipole to London Dispersion
Which intermolecular force is the strongest? Scholars test the relative strength of London dispersion forces, dipole-dipole interactions, and induced dipoles using a simulator. The interactive allows learners to pull on paired molecules...
Concord Consortium
Seeing Intermolecular Attractions
Ahh, the rules of attraction...intermolecular attraction! Introduce your chemistry crew to the other forces that influence the behavior of atoms and molecules alike with a simple interactive. Pupils push and pull polar and non-polar...
CK-12 Foundation
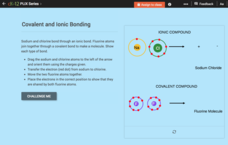
Chemical Bonds: Covalent and Ionic Bonding
Get back to bonding basics. Science scholars get a chance to show what they know using a simple interactive. Pupils create models of covalent and ionic bonds before answering questions about each interaction. The resource includes a...
CK-12 Foundation
Soap
Examine the chemistry of the laundry room! A thorough video explains the polarity and non-polarity properties of soap. The tutorial continues to explain the advantage of these properties in stain removal by showing the bonding of the...