Teach Engineering
Red Cabbage Chemistry
Using the natural pH indicator of red cabbage juice, groups determine the pH of different everyday liquids. As they work, pupils gain an understanding of pH that may help deal with contaminants in the water supply.
It's About Time
Properties of Matter
Never trust an atom; they make up everything! Young chemists make modeling dough and add another ingredient to change the properties. Scholars then compare the properties of emulsion to composite materials. A reading passage and analysis...
Teach Engineering
Balancing Liquid on a Coin: How Intermolecular Forces Work
Let knowledge of chemistry flow like water. Future scientists conduct two different experiments to investigate the properties of water. They learn about surface tension and cohesion as they see how many drops of water they can place on a...
Teach Engineering
Investigating Contact Angle
Discover the properties of water-loving and water-hating surfaces. In the seventh installment of a nine-part series, scholars explore hydrophilic and hydrophobic surfaces by conducting an experiment. They observe surface coatings,...
It's About Time
Elements and Compounds
Young scientists use electrolysis to separate water into its elements before experimenting with fire to learn about their properties. A helpful resource provides a reading passage and analysis questions.
Exploratorium
Indicating Electrolysis
Sure, your learners know water is made up of two molecules, but watching them separate helps the class see the construction like never before. This resource provides directions on how to build a simple electrolysis device using a...
Teach Engineering
Battle of the Beams
Make the strongest beam possible using taffy? Groups mold a taffy-water mixture into a beam and a reinforcing material of their choice. To finish the final installment of a two-part series, participants test its strength by adding...
American Chemical Society
Liquids - Clearly Unique
Bring chemistry to life for scholars as they perform two tests to examine the unique properties of three liquids. Classroom investigators make observations, develop basic lab skills, and follow step-by-step instructions to compare water,...
National Nanotechnology Infrastructure Network
Can Small Pollutants Harm Aquatic Organisms?
Nanoparticles have toxic effects on plant and animal life—even though you can't see them. The second instructional activity of a two-part series has young scientists conduct an experiment that exposes plant and animals to nanoparticle...
Cornell University
Study Soil
What's in soil? Young scientists study the pH levels of soil from their school yard. They observe the land and area the soil came from to decide if location has anything to do with acidity level.
TeachEngineering
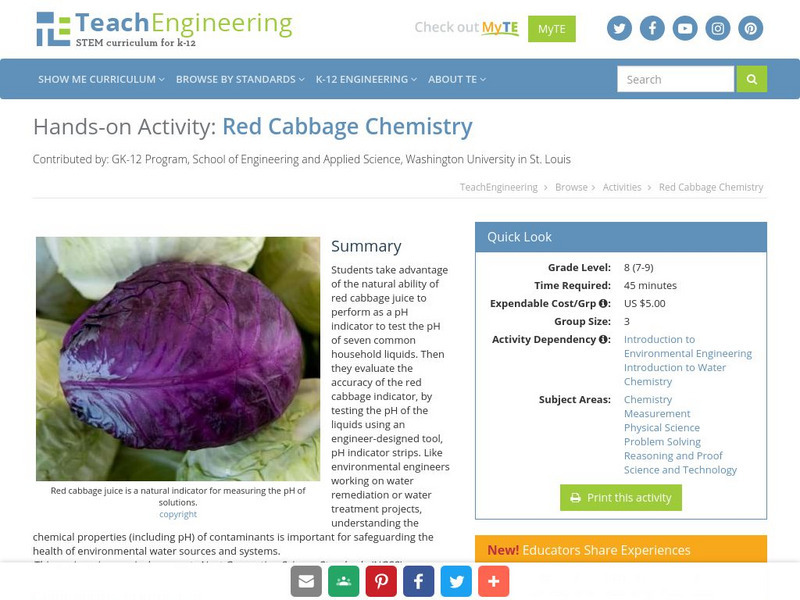
Teach Engineering: Red Cabbage Chemistry
Students take advantage of the natural ability of red cabbage juice to perform as a pH indicator to test the pH of seven common household liquids. Then they evaluate the accuracy of the red cabbage indicator, by testing the pH of the...
University of Maryland
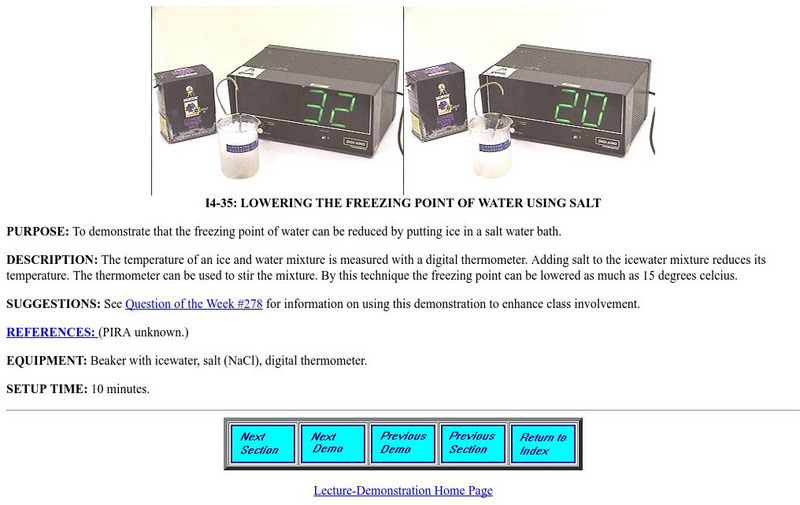
Lowering the Freezing Point of Water Using Salt
A page from the University of Maryland Physics Lecture Demonstration Facility. Provides directions for a teacher demonstration of the effect of an ionic solute upon the freezing point of water. Shows apparatus and set-up; provides...
Science Buddies
Science Buddies: Saturated Solutions: Measuring Solubility
Many essential chemical reactions and natural biochemical processes occur in liquid solutions, so understanding the chemical properties of liquid solutions is fundamentally important. This project will challenge you to discover how much...
Other
Science House: Ice Cream
Experiment shows students how to use the lowered freezing point of water to chill another mixture (ice cream) to the solid state. Teacher's notes provide background information.