Creative Chemistry
Preparation of Crystalline Derivatives of Aldehydes and Ketones
Chemistry explorers prepare a crystalline derivative and find its melting point. Once they discover the melting point, they can identify whether the substance is pure or an aldehyde or ketone. This outstanding laboratory activity helps...
Curated OER
Condensation Polymerization
This organic chemistry lab activity is appropriate for teaching polymerization, percent yield, melting point, or the types and uses of polymer materials. Chemistry pupils imagine that they are working for a company to develop a special...
Santa Monica College
Introducing Measurements in the Laboratory
We use basic units of measurement to break down things and communicate clearly. The first lesson in an 11-part series teaches the proper way to measure various items. It starts simply with measuring the dimensions and areas of geometric...
Curated OER
Condensation Polymerization: Preparation of Two Types of Polyesters
College-level or AP chemists use phthalic anhydride to synthesize two different polyesters, one linear and one cross-linked in structure. A detailed materials list and well-written procedures are provided on a lab sheet. Learners write...
Curated OER
Physical Properties of Matter
Five fabulous procedures introduce physics or chemistry classes to special properties of matter. They discover adhesion and cohesion, solubility, melting and boiling points, and viscosity through hands-on experiences. Tests are...
University of Waikato
Melting Glacial Ice
There are many factors that affect how fast the glaciers are melting. A lab investigation has learners examine how the surrounding water affect the rate glaciers melt. They collect data from two samples of ice to determine how quickly...
Pingry School
Effect of Solutes on Boiling Point
Anyone that lives around snow knows that adding salts to water increases its melting point. Are there solutes that affect the boiling point as well? A scientific experiment has learners add different solutes to water and then...
Curated OER
Ice Cream Lab
I scream, you scream, we all scream for ice cream! Even high schoolers enjoy making ice cream. This laboratory exercise has them record the temperature changes throughout the process of liquid becoming solid, graph the results, and...
Royal Society of Chemistry
Chemistry Masterclass—Chemistry Outreach
Immerse your chemistry class in the world of organic chemistry! Science scholars isolate acetaminophen from an over-the-counter sample during an intense and interesting lab. Groups use many different separation and analysis techniques to...
Curated OER
Energy and States of Matter
Characteristics of each state of matter are listed, the formulas for heating water are detailed, and values for boiling and heat of vaporization are presented. This simple slide show provides direct instruction as well as practice...
Curated OER
Phase Changes of Water
A micro-unit on the phase changes of water includes three laboratory activities. Junior scientists compare the densities of ice and water, and then they do the same for cold and warm water. They examine freezing and boiling temperatures....
Curated OER
The Frigid Gourmet
Students make a " science surprise" or ice cream in the lab. In this melting point depression lesson plan, students use various substances with ice to determine the effects on the melting point of ice and the ability to make ice cream....
Concord Consortium
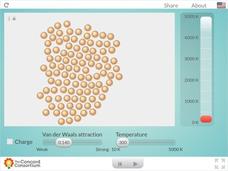
Charged and Neutral Atoms
Do charged and neutral particles behave differently as they undergo phase changes? Science sleuths examine two types of attractive forces using an informative interactive. Pupils can vary the amount of Van der Waals attraction present...
Normal Community High School
Golf Ball Lab
The first golf balls were made of wood and would only last for a few games. Modern golf balls last a lot longer but they don't float. The presentation provides the directions for a lab to determine the minimum amount of salt needed to...
Royal Society of Chemistry
Aspirin—The Wonder of Medicine
What do aspirin and the willow tree have in common? Scholars of chemical synthesis engage in a fascinating reaction to make their own aspirin samples. The lab uses thin layer chromatography analysis, includes stoichiometric calculations,...
Concord Consortium
Seeing Specific Heat and Latent Heat
What happens inside a melting solid? Prospective physical chemists observe a solid-to-liquid phase change at the molecular level using an inspired interactive. Pupils add heat to a close system, then monitor changes in kinetic and...
Curated OER
Macromolecule Lab
During a macromolecule lab, young chemists perform multiple tests, including iodine starch tests, to determine if eight mystery foods contain lipids, sugars, or starches.
Hazardous Waste Management Program
Molar Mass Determination from Freezing Point Depression
Let the data tell the story. A lab activity has pupils collect temperature data on solutes as they melt and freeze. They use their data to create a cooling curve and then calculate the molar mass using the freezing point depression.
Curated OER
Ice Cream Lab
This very thorough lesson plan has learners collect all ingredients necessary and work in pairs to make ice cream using plastic bags. They take temperature readings and record them on the chart as well as record findings on the...
Royal Society of Chemistry
Organic Molecules Day—Chemistry Outreach
In search of an organic lab that employs real-life techniques and analysis methods? Groups carry out the nitration of methyl benzoate, then attempt to determine the number and location of the nitro groups added to the benzene ring....
Curated OER
The Day After Tomorrow: How is the Density of Water Related to Climate Change and Global Warming?
Science learners simulate what happens when ice breaks up and floats on water and how increased pressure on ice causes it to melt faster. They view a clip from the movie, The Day After Tomorrow, and relate their lab activities to what...
Concord Consortium
Specific Heat and Latent Heat in Condensation
There's more to melting than meets the eye! Junior physical chemists investigate the differences between specific and latent heats as a substance undergoes a phase change. Users remove heat from the system and observe changes in kinetic...
Curated OER
Ice Cream: a Taste of Science!!
Students define the term solution. They explain conservation of energy and energy transfer as it relate to how the milk solution became ice cream. Students are able to explain freezing point depression.
Curated OER
Types of Bonding Lab
Chemistry lab learners experiment to identify a series of unknown compounds. They choose which tests to perform, but you may suggest melting or boiling point, solubility, electrical conductivity, and malleability. This is a terrific...