American Chemical Society
Changing State: Melting
Dry ice is extremely cold — it is -109.3°F or -78.5°C. Scholars observe and explain the molecular motion associated with melting. Then they design their own experiments to speed up the melting process. Finally, a teacher presents a...
University of Georgia
Freezing and Melting of Water
Examine the behavior of energy as water freezes and melts. An engaging activity provides a hands-on experience to learners. Collaborative groups collect data and analyze the graphs of the temperature of water as it freezes and then...
Santa Monica College
Introducing Measurements in the Laboratory
We use basic units of measurement to break down things and communicate clearly. The first lesson in an 11-part series teaches the proper way to measure various items. It starts simply with measuring the dimensions and areas of geometric...
Pingry School
Effect of Solutes on Boiling Point
Anyone that lives around snow knows that adding salts to water increases its melting point. Are there solutes that affect the boiling point as well? A scientific experiment has learners add different solutes to water and then...
Nevada Outdoor School
Let It Snow! Let It Melt!
Winter weather offers a great opportunity to teach young scientists about the states of matter. This activity-based lesson includes a range of learning experiences, from experimenting with the rate at which ice melts...
University of Waikato
Melting Glacial Ice
There are many factors that affect how fast the glaciers are melting. A lab investigation has learners examine how the surrounding water affect the rate glaciers melt. They collect data from two samples of ice to determine how quickly...
Messenger Education
Design Challenge: How to Keep Gelatin from Melting
The inside of the spacecraft Messenger, which explores Mercury, will experience temperatures from 32 to 91 degrees Fahrenheit. In the final installment of a series of four space-related activities, groups spend time discussing and...
Concord Consortium
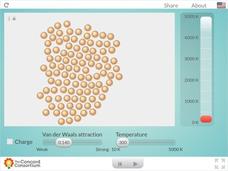
Charged and Neutral Atoms
Do charged and neutral particles behave differently as they undergo phase changes? Science sleuths examine two types of attractive forces using an informative interactive. Pupils can vary the amount of Van der Waals attraction present...
Hazardous Waste Management Program
Molar Mass Determination from Freezing Point Depression
Let the data tell the story. A lab activity has pupils collect temperature data on solutes as they melt and freeze. They use their data to create a cooling curve and then calculate the molar mass using the freezing point depression.
Concord Consortium
Seeing Specific Heat and Latent Heat
What happens inside a melting solid? Prospective physical chemists observe a solid-to-liquid phase change at the molecular level using an inspired interactive. Pupils add heat to a close system, then monitor changes in kinetic and...
Resources for Educators
Fractions of Fun
Reinforce concepts and encourage learner engagement with a collection of math games, science experiments, and cross curricular activities. In one fun resource, learners sort objects, keep a diary of everyday fractions, play a game using...
Royal Society of Chemistry
Chemistry Masterclass—Chemistry Outreach
Immerse your chemistry class in the world of organic chemistry! Science scholars isolate acetaminophen from an over-the-counter sample during an intense and interesting lab. Groups use many different separation and analysis techniques to...
Concord Consortium
Specific Heat and Latent Heat in Condensation
There's more to melting than meets the eye! Junior physical chemists investigate the differences between specific and latent heats as a substance undergoes a phase change. Users remove heat from the system and observe changes in kinetic...
American Chemical Society
Changes Caused by Heating and Cooling
It's heating up—and cooling down—in here! A hands-on lesson allows learners to experiment with melting and freezing butter to observe changes as a substance transitions between liquid and solid form. They also view an animation that...
Royal Society of Chemistry
Symbols
Chemistry calculations can look a bit like alphabet soup at times. How do you help pupils make sense of it all? An interactive resource helps scholars sort through the symbols for common quantities such as moles, boiling point, and...
Teach Engineering
All Fats Are Not Created Equal
Apply robotics to connect physical properties to chemical properties. Future engineers use robots to determine the melting points of various fats and oils. The robots can do this by measuring the translucency of the fats as they heat up.
DiscoverE
Ice Cream Special
We all scream for ice cream! Individuals create home-made ice cream in the classroom. This is a delicious way to show a real-world application of the freezing point depression to your class.
Teach Engineering
Concentrate This! Sugar or Salt...
Heat up your lessons on boiling points. The resource provides a three-part activity: first, groups find the boiling point of solutions; second, they create boiling point curves for salt and sugar solutions; and third, they mix a solution...
Royal Society of Chemistry
Aspirin—The Wonder of Medicine
What do aspirin and the willow tree have in common? Scholars of chemical synthesis engage in a fascinating reaction to make their own aspirin samples. The lab uses thin layer chromatography analysis, includes stoichiometric calculations,...
Normal Community High School
Golf Ball Lab
The first golf balls were made of wood and would only last for a few games. Modern golf balls last a lot longer but they don't float. The presentation provides the directions for a lab to determine the minimum amount of salt needed to...
Center for Learning in Action
Water – Changing States (Part 2)
Here is part two of a two-part lesson in which scholars investigate the changing states of water—liquid, solid, and gas—and how energy from heat changes its molecules. With grand conversation, two demonstrations, and one hands-on...
Beyond Benign
Enthalpy of Combustion
Learn the facts about types of wax! Partnered pupils determine the enthalpy of combustion for traditional paraffin candles, as well as soy-based candles. The activity focuses on calculations and compares the environmental impact of both...
Royal Society of Chemistry
Alloys
What are alloys, and why do we use them? Through a series of interactive puzzles, scholars examine the components and uses of several common alloys. The accompanying teacher's resources provide support in using the lesson, printable...
Royal Society of Chemistry
Organic Molecules Day—Chemistry Outreach
In search of an organic lab that employs real-life techniques and analysis methods? Groups carry out the nitration of methyl benzoate, then attempt to determine the number and location of the nitro groups added to the benzene ring....
Other popular searches
- Boiling Point Melting Point
- Freezing Melting Point
- Melting Point Lab
- Melting Point Pure
- Boiling Point, Melting Point
- Ice Melting Point
- Freezing and Melting Points
- The Melting Point
- Melting Point Pure Mix