Curated Video
Reaction Rates Unlocked : Definition, Explanation, Analogies, and Applications
Reaction Rates Unlocked : Definition, Explanation, Analogies, and Applications Rate of Reaction and Instantaneous Rate part 1
Professor Dave Explains
Kinetics: Initial Rates and Integrated Rate Laws
Who likes math! Oh, you don't? Maybe skip this one. Unless you have to answer this stuff for class. Then yeah, watch this.
TED-Ed
How to Speed Up Chemical Reactions (and Get a Date)
How are chemical reactions like dating? A collision must first occur! In this hilarious approach to speeding up chemical reactions, viewers find out that five changes can increase the rate of reaction: smaller space, increased number of...
TED-Ed
If Molecules Were People...
By watching this droll and delightful animation, physical scientists consider what happens when molecules collide. In this film, however, parodic people bump into each other, exchanging limbs in the process, just as molecules might trade...
GPB Television
Chemistry 1201: Reaction Rates
Mr. Mole and Mr. Matchmaker entertain and educate your chemists about how quickly or slowly reactants are used up in a chemical reaction. Thorough explanations of the influencing factors are offered, and demonstrations are done. After...
JFR Science
Reaction Mechanisms
Why do chemical reactions move at different rates? Chemistry scholars examine the intricate workings of reaction mechanisms with a video from the JFR Science playlist. They watch as the narrator diagrams reaction intermediates and...
JFR Science
Introduction to Rates of Reaction
Why are rates of reaction so important? Discover the answer to this, and many other questions, through a video from JFR Science. Pupils examine how, through the balanced chemical equation and experimentation, they can determine how fast...
Teacher's Pet
Factors Affecting Reaction Rates
You can speed up or slow down a reaction when you know these factors. Learners explore the factors affecting reaction rates such as catalysts and inhibitors, temperature, surface area, and concentration. The video provides examples for...
Teacher's Pet
Intro to Equilibrium
Now that chemistry scholars are comfortable with reaction types, shake things up with a video about equilibrium. Topics include reversible reactions, equilibrium within a sample of water, and conditions that force either more products or...
Fuse School
Rates of Reactions—Part 1
What does a reaction rate tell you about your reaction? A thorough video lesson explains how to measure and read a reaction rate using two different methods. As the fifth installment in a 35-part chemistry series, the lesson uses...
Fuse School
Green Chemistry - Principle 8
Energy conservation is a global effort, but how can chemists aid the cause? The narrator in the eighth installment of a 12-part Green Chemistry series describes the importance of reducing reactant waste while performing reactions with...
Fuse School
Green Chemistry - Principle 6
Using a catalyst to reduce reaction times is often necessary for a reaction to be profitable. But are all catalysts safe? The sixth installment in a 12-part Green Chemistry series has learners explore the role of catalysts in industrial...
Fuse School
Rates of Reactions—Part 2
Learners examine the factors that affect reaction rates in the sixth lesson in a 35-part video series. The video instructor explains the different factors that change reaction rates, and the connection between volume, pressure, and...
Khan Academy
Khan Academy: Energy and Enzymes: Introduction to Kinetics
This video discussed kinetics which is the rate of the reactions. Learn about activated complexes and activation energy. Learn variables that affect the rate of reaction like adding a catalyst, increasing the concentration of molecules,...
Khan Academy
Khan Academy: Rate of Reaction
Definition of reaction rate, and examples of calculating the average rate of reaction. [9:09]
Khan Academy
Khan Academy: Finding Units of Rate Constant K
Discover how to find the units for the rate constant k for a zero, first, or second order reaction. [5:05]
Khan Academy
Khan Academy: Plotting Data for a First Order Reaction
Identify an example of graphing first-order rate data to see a linear relationship and calculating rate constant k from the slope. [9:13]
Khan Academy
Khan Academy: Rate Constant K From Half Life Example
An example problem shows how to find the rate constant k from the half-life of a first-order reaction. [6:10]
Khan Academy
Khan Academy: Plotting Data for a Second Order Reaction
An example of graphing second order rate data to see a linear relationship, and calculating rate constant k from the slope is introduced from Khan Academy. [8:02]
Khan Academy
Khan Academy: Zero Order Reaction (With Calculus)
Understand the process of deriving the integrated rate law for zero-order reactions using calculus. Identify how to graph zero order rate data to see a linear relationship. [9:46]
Bozeman Science
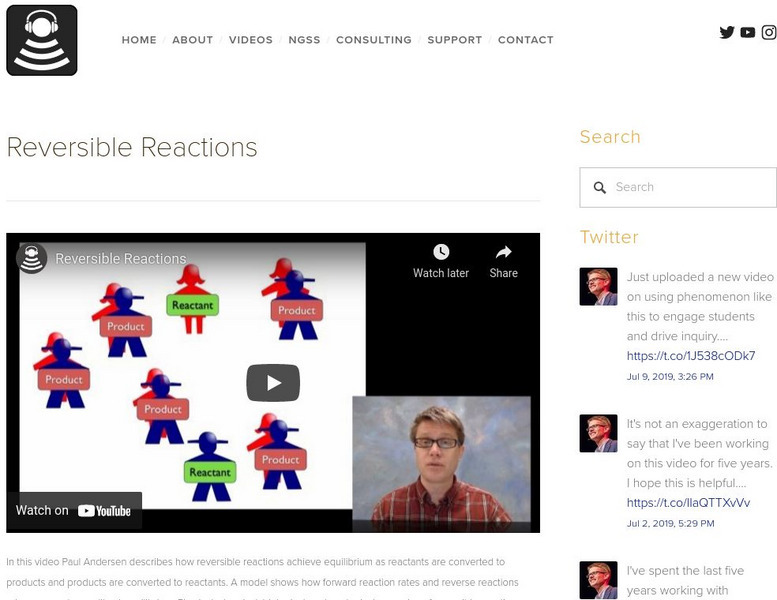
Bozeman Science: Reversible Reactions
In this video Paul Andersen describes how reversible reactions achieve equilibrium as reactants are converted to products and products are converted to reactants. A model shows how forward reaction rates and reverse reactions rates...
Bozeman Science
Bozeman Science: Elementary Reactions
In this video Paul Andersen explains that elementary reactions are steps within a larger reaction mechanism. Colliding molecules require sufficient energy and proper orientation to break bonds and form new bonds. A unimolecular reaction...
Bozeman Science
Bozeman Science: The Rate Constant
In this video Paul Andersen describes the characteristics of the rate constant in chemical reactions. The rate constant is highly variable in reactions and must be determined experimentally. The rate constant is dependent on both...
Bozeman Science
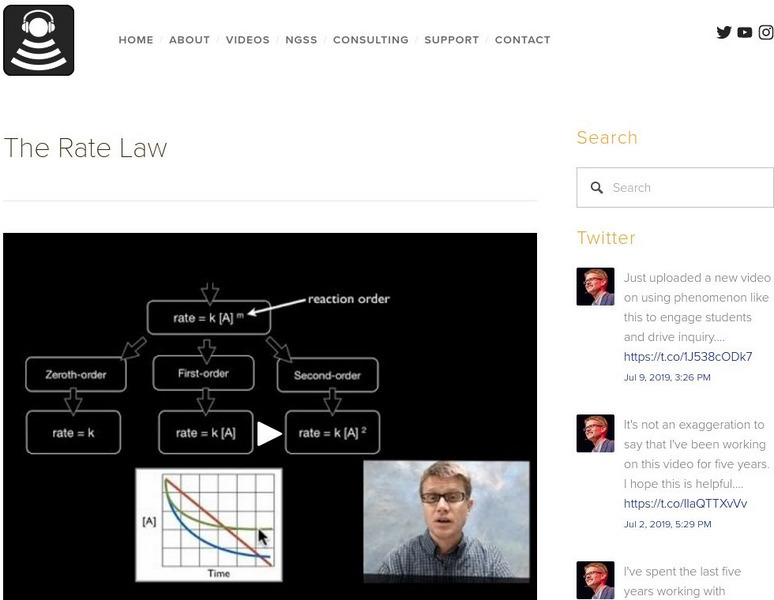
Bozeman Science: The Rate Law
Paul Andersen explains how the rate law can be used to determined the speed of a reaction over time. Zeroth-order, first-order and second-order reactions are described as well as the overall rate law of a reaction. The rate of a reaction...