Curated OER
Graham's Laws: Diffusion and Effusion of Gases
Students conduct a series of experiments to explore Graham's law. In this chemistry lesson, students differentiate effusion and diffusion. They perform calculations using Boyle's, Henry's, Charles' and Graham's Laws.
Curated OER
Graham's Law
In this gas laws activity, students apply Graham's Law by completing 6 problems finding the velocity and molecular mass of gases.
Curated OER
Graham's Law
In this gas law activity, students learn how Graham's law governs the diffusion of gases. Students calculate the velocity and effusion rate of gases at certain temperatures. This activity has 6 problems to solve.
Curated OER
Partial Pressure and the Kinetic Molecular Theory of Gases
In this pressure and kinetic theory worksheet, students review Dalton's Law of Partial Pressure, the Kinetic Molecular Theory of Gases, and Graham's Law of Effusion. Students then solve 5 problems using these equations.
Science Geek
Kinetic Molecular Theory
The fourth presentation in a series of five begins with information about the nature of gases and what to expect from them. Then it discusses kinetic molecular theory, diffusion, and Graham's Law, and concludes with the purification of...
CK-12 Foundation
Fish vs. Rose
Examine the science behind rates of diffusion. The video lesson and following interactive activity focus on the molecular components of different smells. Learners monitor the diffusion of each smell as they 'race' through a room.
Curated OER
The Great Gas Race
Fourth graders improve their understanding of Graham's Law by using properties of gases to evaluate the rate of effusion of two compounds as they vaporize. They engage in a lab which elevates their understanding of the properties of gases.
Chem Tutor
Chem Tutor: Graham's Law of Diffusion (Or Effusion)
A decent site with a good explanation of Graham's Law of diffusion. Includes the formula for this law and some sample problems at the bottom of the page.
Mocomi & Anibrain Digital Technologies
Mocomi: What Is Graham's Law?
Definitions, formulas, and fun facts for Graham's Law, Ideal Gas Law, and the Kinetic Molecular Theory.
Simon Fraser University
Chem1 Virtual Textbook: Effusion and Graham's Law
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with both effusion and its relation to Graham's law.
University of Nebraska
Do Chem: Graham's Law
A lab setup, fairly simple, to illustrate Graham's Law. Check it out!
CK-12 Foundation
Ck 12: Gas Mixtures and Molecular Speeds
[Free Registration/Login may be required to access all resource tools.] The following online tutorial helps students use Dalton's Law and mole fraction to calculate the partial pressure of a gas in a mixture. They will learn to calculate...
Other
South Carolina University: Effusion and Diffusion
Graphic from a slide show shows the concept of effusion nicely. Definitions included.
CK-12 Foundation
Ck 12: Chemistry Simulation: Speedy Smells
[Free Registration/Login Required] Explore how the mass of a molecule affects how quickly a smell will reach our noses.
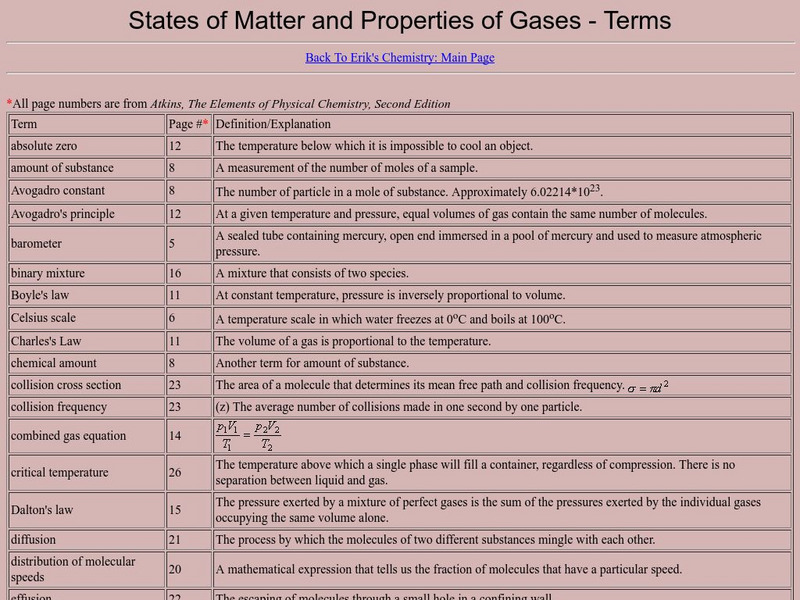
Other
States of Matter and Properties of Gases: Terms
A very complete list of terms that are important to the study of gases. This resource is a web archive.