Avogadro's hypothesis Teacher Resources
Find Avogadro's hypothesis lesson plans and worksheets
Showing 67 resources
OpenStax
Open Stax: College Physics: The Ideal Gas Law
A section in a college textbook exploring the ideal gas law in relation to molecules and in terms of moles. Learn how to calculate the variables in the ideal gas law. Also become familiar with Avogadro's number and how to use it to...
Texas Education Agency
Texas Gateway: The Ideal Gas Law
Explore the thermal behavior of gases, in particular, examine the characteristics of atoms and molecules that compose gases. Learn about the ideal law of gases in terms of molecules and moles. Multiple formulas and examples are provided.
Georgia State University
Georgia State University: Hyper Physics: Ideal Gas Law
This site defines and discusses the ideal gas law. The concept of state variables is explained and the various state variables are identified. Links to further information is available.
Texas Education Agency
Texas Gateway: Temperature, Kinetic Theory, and the Gas Laws: Glossary
This is a glossary of terms and definitions used in Chapter 13: Temperature, Kinetic Theory, and the Gas Laws from the AP Physics online text.
CK-12 Foundation
Ck 12: The Ideal Gas Law
[Free Registration/Login may be required to access all resource tools.] Students read about and explore video clips, diagrams, and example problems using the Ideal Gas Law.
Khan Academy
Khan Academy: Thermodynamics Part 5: Molar Ideal Gas Law Problem
Sal uses the molar version of the ideal gas law to solve for the number of moles in a gas. He also shows how to convert this answer into number of molecules using Avogadro's number. [8:01]
Simon Fraser University
Chem1 Virtual Textbook: Avogadro's Law
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with a variety of topics related to real gases including effects of...
University of Florida
Chemistry 2041 Lecture Notes: Ideal Gases
The ideal gas law is presented and explained. The derivation of other gas laws is performed. Gas behavior is explained in terms of gas laws. Excellent graphics.
Towson University
Towson University: Ideal and Real Gas Laws
The ideal gas law is stated and explained at this site from the Towson University. It is then used to derive the other gas laws (Charles, Boyle's, Gay-Lussac's, Avogadro's, combined, etc.). Other gas law relationships are discussed....
Wolfram Research
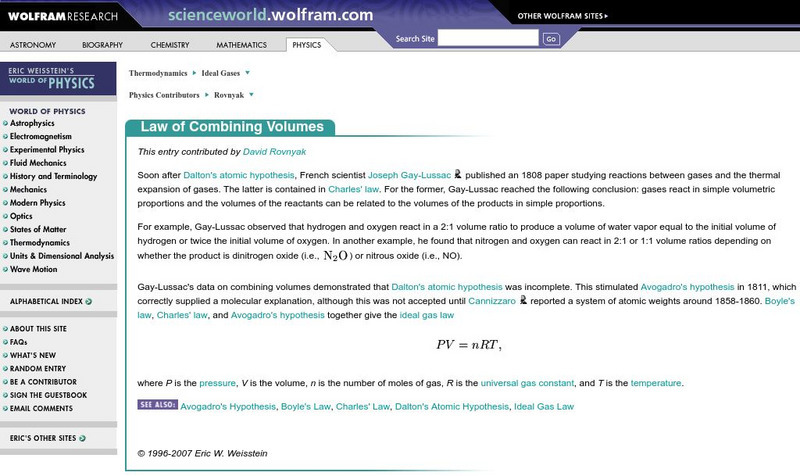
Wolfram Science World: Law of Combining Volumes
This site contains informaiton on the law of combining volumes. A formula is given and links to key terms.
ClassFlow
Class Flow: Gas Laws
[Free Registration/Login Required] This flipchart covers core concepts of Gas Laws for High School Chemistry. Topics covered are Boyles Law, Charles, Gay Lussac, Combined Gas Law, Avogadro volume, Temperature Conversion between Kelvin...
Michael Blaber, PhD
Florida State University: Gases: Gas Mixtures & Partial Pressure
Avogadro's law is stated in equation form. The lesson does not include much discussion, but it does relate the partial pressure and the total pressure of a gas sample to the mole fraction of its components.
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Gases
This chemistry e-textbook provides students with AP-level reading and practice material on gas laws.
University of Sydney (Australia)
Thermal Physics Module: Ideal Gases [Pdf]
A molecular model of a gas is introduced and explained. Assumptions behind the ideal gas law are presented. The ideal gas law is stated. Charles' law and Boyle's law are derived from the ideal gas law.
Wyzant
Wyzant: Chem Tutor: Gases
A lengthy page covering most all the gas laws. Each law is described in words and stated as an equation. The use of each law in solving problems is demonstrated. Some problem-solving tips are provided. There are 23 practice problems with...
Frostburg State University
General Chemistry Online: Gases Faq
Float away with the answers to many frequently asked questions about gases. Understand Avogadro, reaction stoichiometry and more.
Khan Academy
Khan Academy: Thermodynamics: Thermodynamics (Part 5)
Demonstrates an example problem involving the formula PV=nRT. After calculating how many moles, he uses Avogadro's Number of molecules per mole, to calculate how many atoms that amount would represent. [8:00in]
Nobel Media AB
The Nobel Prize: Jean Baptiste Perrin Nobel Lecture
At this site from The Nobel Foundation you can read Jean Baptiste Perrin's Nobel Lecture, "Discontinuous structure of matter," in which Perrin overviews the work which earned him the Nobel Prize in Physics.
Other
States of Matter and Properties of Gases: Terms
A very complete list of terms that are important to the study of gases. This resource is a web archive.












![Thermal Physics Module: Ideal Gases [Pdf] Handout Thermal Physics Module: Ideal Gases [Pdf] Handout](https://d15y2dacu3jp90.cloudfront.net/images/attachment_defaults/resource/large/FPO-knovation.png)