Frostburg State University
General Chemistry Online: Laboratory Operations Faq
A narrow list of questions about technicalities with chemistry labs. Find out the how to properly clean glassware and why acid should always be added to water in that order.
Frostburg State University
Frostburg State University: Antoine: Chemistry: Matter
A slideshow illustrating the science of matter from its properties, states, and classification to mixtures and the Periodic Table of Elements.
Frostburg State University
General Chemistry Online: Limiting Reactant Problem
Explains the essence of limiting reactant problems using a sample problem: How can an amount of product (KNO3) be predicted from amounts for two reactants (KCl and HNO3)? Shows the work and explains how to solve these types of problems.
Frostburg State University
General Chemistry: Extensive and Intensive Properties
Provides information about extensive and intensive properties of matter. Gives examples of these properties. Includes a link to a quiz on classifying properties.
Frostburg State University
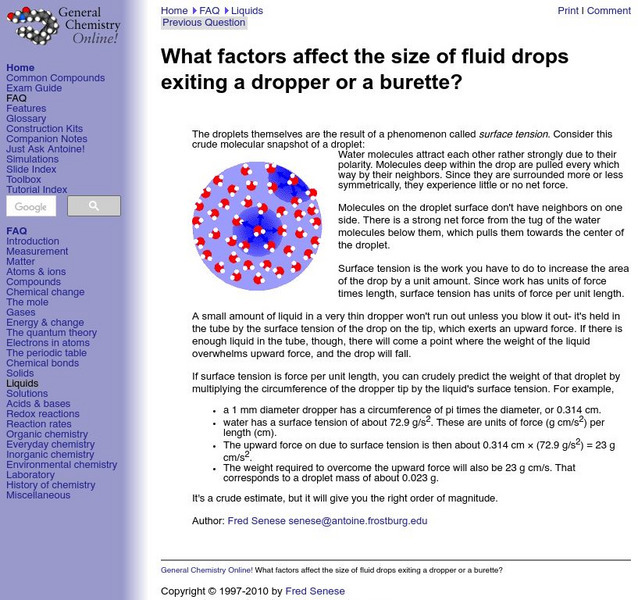
Frostberg University: Surface Tension and Droplet Size
Discusses the role of surface tension in determining the size of water droplets. Explanation of why a small amount of liquid in a dropper will never run out.
Frostburg State University
General Chemistry Online: Rules for Significant Digits
This site from General Chemistry Online provides a mnemonic device you may not have seen to help remember the rules for significant digits called the Atlantic-Pacific Method. Also includes a comparison between this rule and the...
Frostburg State University
General Chemistry Online: Dalton's Atomic Theory
Discusses John Dalton's development of the atomic theory and the four main points of his theory. Includes references and a link to a quiz on Dalton's Atomic theory.
Frostburg State University
Frostburg State University: Limiting Reagents
Middle of a slide show on stoichiometry that shows a nice graphical look and example of limiting reagents.
Frostburg State University
General Chemistry Online: Who Was Lothar Meyer?
A short description of Lothar Meyer, one of the men who discovered the periodic table. There is a link to his original periodic table.
Frostburg State University
General Chemistry Online: Reactions in Solution
Resource provides a set of 18 slides that discuss chemical reactions in solution. Slides 15-18 discuss metathesis (double replacement) reactions, including formation of insoluble precipitate.
Frostburg State University
General Chemistry Online: Heterogeneous Mixtures
This is a brief definition from Frostburg State University of a heterogeneous mixture. Includes commonly known examples of these mixtures.
Frostburg State University
Frostburg State University: How Does H2 Co3 Form When Co2 Dissolves in Water?
A brief explanation of how carbonic acid forms when carbon dioxide dissolves in water.
Frostburg State University
General Chemistry Online: What Is the Buffer in Buffered Aspirin
This site from Frostburg State University gives the structural formula of aspirin as well as some information about its biochemical interaction in the stomach.
Frostburg State University
General Chemistry Online: What Is a Valence Bond?
Brief explanation from Frostburg State University of valence bond theory and an explanation of what chemical situation creates a valence bond between two atoms.
Frostburg State University
Frostburg State General Chemistry Online: What Is a Non Newtonian Fluid?
Describes viscosity and its relationship to Newtonian and Non-Newtonian liquids. Gives examples in terms of everyday substances.
Frostburg State University
Frostburg State Chemistry Online: Pressure & Oxygen Solubility
The effect of changing pressure on the solubility of oxygen in water. Explains how temperature and pressure affect dissolved oxygen levels. Includes example formulas.
Frostburg State University
General Chemistry Online: Estimating Freezing Point
This site from the General Chemistry Online of the Frostburg State University provides informaiton on estimating freezing point of a solution from freezing point constant and molality.
Frostburg State University
Frostburg State University: Miscible & Immiscible Liquids
Discussion of miscible (mixable) and immicible (unmixable) liquids.
Frostburg State University
Frostburg State Univ.: Test for Sucrose
Frostburg State University provides a chemical procedure for determining the presence of a sucrose.
Frostburg State University
University of Frostburg: Dissociation of Water
Discussion of dissociation (self-ionization) of water and the reasons why hydrogen does not dissociate in water.
Frostburg State University
Frostburg State General Chemistry Online: Ionic Dissociation
Slides six and seven of a lecture series illustrate and explain ionic dissociation. These slides are in a group of nineteen slides about chemical reaction in solution.
Frostburg State University
Frostburg State University: Glossary Solutions
A glossary of terms related to solutions.
Frostburg State University
General Chemistry Online!: Why Are Acids Called Proton Donors?
This page provides a concise explanation of why acids are called proton donors.
Frostburg State University
General Chemistry Online: Lipstick Chemistry
This site from Frostburg State University answers a dye relevant question and in doing so gives the structural formula of a common dye. It explains how lipstick color lasts, and what chemical produces the red color.