Hi, what do you want to do?
Science Struck
Science Struck: Explanation of John Dalton's Atomic Theory
Explains the basic tenets of Dalton's atomic theory.
CK-12 Foundation
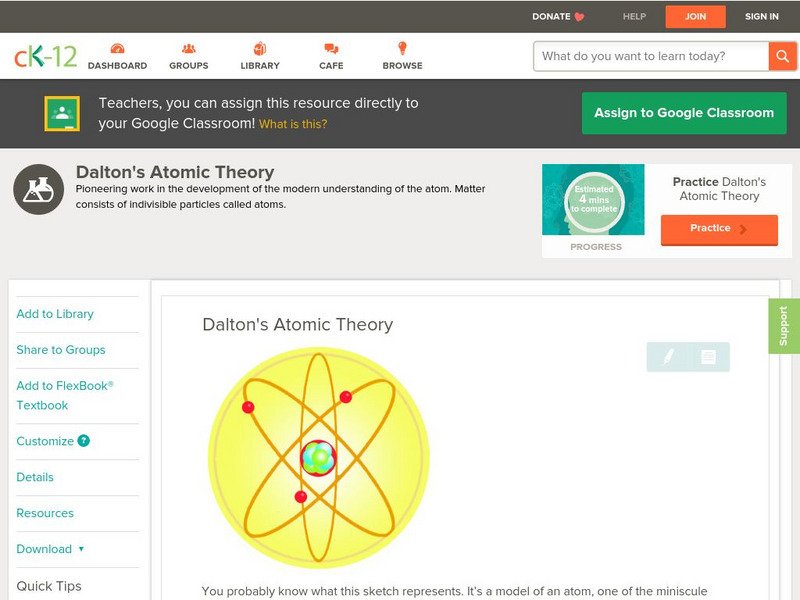
Ck 12: Physical Science: Dalton's Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Reintroduction of the idea of the atom and the billiard ball model.
Nobel Media AB
The Nobel Prize: The Nobel Peace Prize 1962
Read about Linus Carl Pauling, the winner of the 1962 Nobel Peace Prize. This website is organized into the following sections: "Presentation Speech," "Biography," "Nobel Lecture," "Other Resources," and "The Nobel Chemistry Prize 1954."
Texas Education Agency
Texas Gateway: Atoms, Elements and the Periodic Table: The Atomic Model [Pdf]
A slideshow looking at the contributions of scientists over time to our understanding of atomic theory. Looks at models of the atom developed by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick, as...
Upper Canada District School Board
Tom Stretton's Chemistry Pages: Atomic Theory Early Ideas
This online slide show helps students understand the early historical thinking that contributed to modern atomic theory.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Modern Atomic Theory: Power Point [Pdf]
This power point highlights the main ideas about modern atomic theory from the online textbook "An Introduction to Chemistry". Step by step instructions are given to write electron configurations and draw orbital diagrams. Also includes...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Modern Atomic Theory: Study Guide [Pdf]
This study guide provides an overview of the modern atomic theory chapter from "An Introduction to Chemistry. Find chapter goals, map, checklist, web resources and the answer key to the chapter review.
Texas Education Agency
Texas Gateway: Matter and Energy: Atomic Structure
This tutorial reviews over the basics of atomic structure.
TED Talks
Ted: Ted Ed: The 2400 Year Search for the Atom
How do we know what matter is made of? The quest for the atom has been a long one, beginning 2,400 years ago with the work of a Greek philosopher and later continued by a Quaker and a few Nobel Prize-winning scientists. Theresa Doud...
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
Science Struck
Science Struck: James Chadwick's Atomic Theory and Its Lasting Impact
Explains how James Chadwick came to develop his atomic theory by building upon the work of other scientists.
CK-12 Foundation
Ck 12: Isotopes and Atomic Mass
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will be asked to define atomic number, mass number and understand how isotopes differ from one another. They will be able...
NASA
Nasa: Reaching for the Stars
Find the latest news on research and development underway to use antimatter as a fuel for space vehicles. (April 12, 1999)
Other
Chemtopics: Development of Modern Atomic Theory [Pdf]
A summary of the achievements of J. J. Thomson, Ernest Rutherford, Niels Bohr, and Erwin Schrodinger.
CK-12 Foundation
Ck 12: Evolution of the Atomic Model
[Free Registration/Login may be required to access all resource tools.] Students take a closer look at how our understanding of the atom has evolved over time.
Texas Education Agency
Texas Gateway: Atomic and Molecular Explanation of Pressure and Temperature
We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. Learn more about Kinetic Theory with the following detailed resource...
OpenStax
Open Stax: Atomic and Molecular Explanation of Pressure and Temperature
A college textbook section that explores the kinetic theory. Learn how the kinetic theory relates to ideal gas law and thermal energy. Section includes problems and questions for the students to answer to ensure understanding. Book can...
Texas Education Agency
Texas Gateway: Atomic and Molecular Explanation of Pressure and Temperature
Learn how to calculate the kinetic energy of a gas molecule, describe the relationship between the temperature of a gas and the kinetic energy of atoms and molecule, and describe the distribution of speeds of molecules in a gas. The...
PBS
Pbs Learning Media: That's My Theory!
Become a game show contestant in this online activity from A Science Odyssey and ask a series of questions to a panel of mystery scientists, using the answers to determine which scientist is Einstein.
Concord Consortium
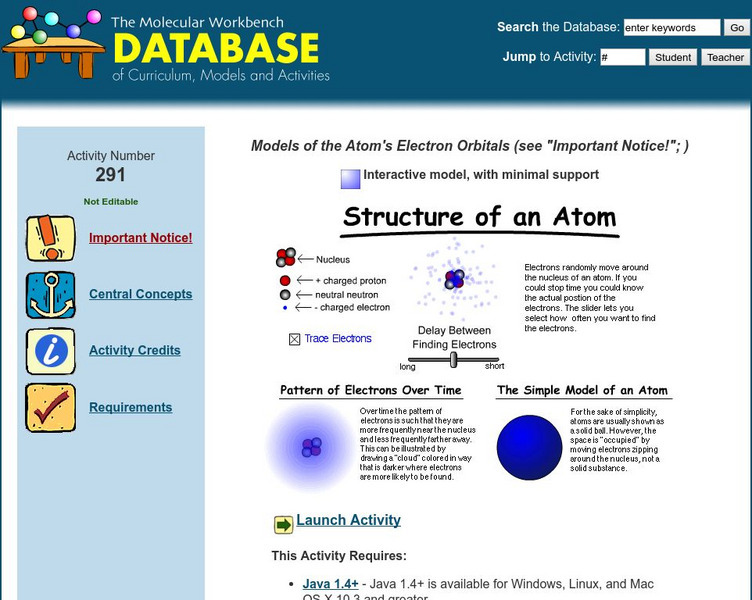
The Molecular Workbench Database: Models of the Atom's Electron Orbitals
Learn about atomic structure and the multiple theories of atomic structure in this simulation.
Nobel Media AB
The Nobel Prize: Erwin Schrodinger Biographical
This biography from the The Nobel Foundation overviews the life and scientific worlk of Erwin Schrodinger, an Austrian scientist who was honored for his work with atomic theory. Read about his education and personal life, and view links...
CK-12 Foundation
Ck 12: Chemistry: Democritus' Idea of the Atom
[Free Registration/Login may be required to access all resource tools.] Explains the ideas of Democritus dealing with the divisibility of the atom.
Nobel Media AB
The Nobel Prize: Niels Bohr Biographical
The Nobel Foundation provides this site about Niels Bohr's contributions to the world of physics, specifically his "investigation of the structure of atoms and of the radiation emanating from them." This biography includes information on...
BBC
Bbc: Gcse Bitesize: Atomic Structure
This lesson focuses on the structure of atoms. All substances are made from atoms. Each atom is made of a nucleus - containing protons and neutrons - surrounded by electrons. It provides a link to an assessment.