Hi, what do you want to do?
Curated OER
Observing the Effect of a Change in Conditions on a System at Equilibrium by Applying Le Chatelier's Principle
Pupils describe the Le Chatelier Principle. They participate in an experiment in which they observe the changes in an equilibrium system. They answer discussion questions to end the lesson.
Curated OER
Activity #14 Floating Bubbles
Students comprehend that Carbon dioxide gas is relatively easy to generate. They comprehend that one way to produce it is with dry ice. Pupils comprehend that carbon dioxide gas can also be produced by combining baking soda with vinegar.
Curated OER
Chlorophyll
Middle schoolers use thin layer chromatography, TLC, to separate various pigments found in plants.
Curated OER
Ice Cream
Students explore the concept of the colligative property. Through experimentation, students lower the freezing point of a liquid in order to create a solid by using household ingredients to create ice cream.
Curated OER
Explorit's Chemistry Quiz
In this chemistry worksheet, students complete a six question multiple choice quiz about chemistry. This is an on-line interactive worksheet.
Curated OER
Striking it Rich with Chemistry
Students identify the composition of different pennies. In this chemistry lesson, students use a post 1982 penny to observe chemical change. They explain how to turn a penny from copper to gold.
Curated OER
Activity #16 Dancing Spagehetti
Students experiment with floating the spaghetti, the gas functions like a life preserver. Pupils comprehend that a person is slightly more dense than water. They comprehend that a life preservers are made of low-density materials. The...
Curated OER
We All Scream for Ice Cream
Students make ice cream while experimenting with the freezing point of water. They experiment with different amounts of salt.
Curated OER
Water Chemistry
Students engage in a lesson that is concerned with the concept of water chemistry. They conduct research using a variety of resources. Students also consider an experiment to observe how water has the abiility to exist as three different...
eSchool Today
E School Today: Elements, Compounds, Substances and Mixtures
Learn about the classification of matter based on whether the chemical composition is pure or a mixture. Understand the definitions of elements, compounds, substances, and mixtures, and what the differences are between them. Five types...
Science Education Resource Center at Carleton College
Serc: Mixtures, Solutions and Saturation: Testing Household Materials
Choosing two different powders, students will evaluate the properties of the materials and if the materials, when mixed with 50 ml of water, make a mixture or solution. They will examine the difference between a mixture and a solution...
Simon Fraser University
Chem1 Virtual Textbook: Solutions [Pdf]
With an overview of seven topics related to solutions, this .pdf file provides information on everything from osmosis and osmotic pressure to ions in aqueous solution. Other topics include solutions of volatile substances, methods of...
Georgia Department of Education
Ga Virtual Learning: Chemistry: Solutions
In this module, students study solutions; how they are formed, how to calculate concentration of solutions, and what the colligative properties of solutions are.
Chem4kids
Chem4 Kids: Matter: Solutions and Mixtures
This Chem4kids tutorial teaches the basic properties of solutions.
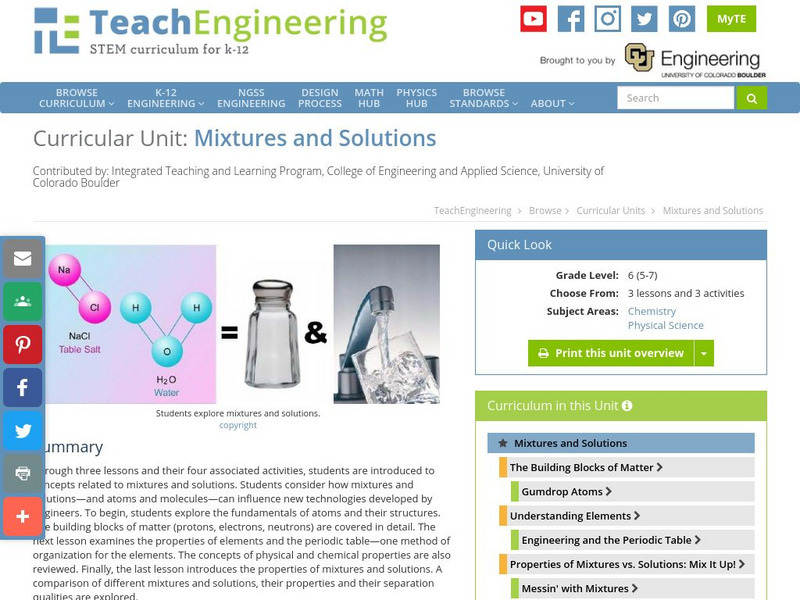
TeachEngineering
Teach Engineering: Mixtures and Solutions
This unit covers introductory concepts of mixtures and solutions. Students think about how mixtures and solutions, and atoms and molecules can influence new technologies developed by engineers. The first lesson explores the fundamentals...
TED Talks
Ted: Ted Ed: Science of Macaroni Salad: What's in a Mixture?
What's in macaroni salad? Break down the pasta, mayonnaise, vinegar, mustard, vegetables, etc., and you're left with a bunch of molecules. Josh Kurz uses a delicious recipe to exemplify three types of mixtures (solution, colloid and...
TeachEngineering
Teach Engineering: Separating Mixtures
In this lesson the students will learn how to classify the materials as mixtures, elements and compounds and identify the properties of each group. Also the concept of separation of mixtures will be introduced to the students. Since...
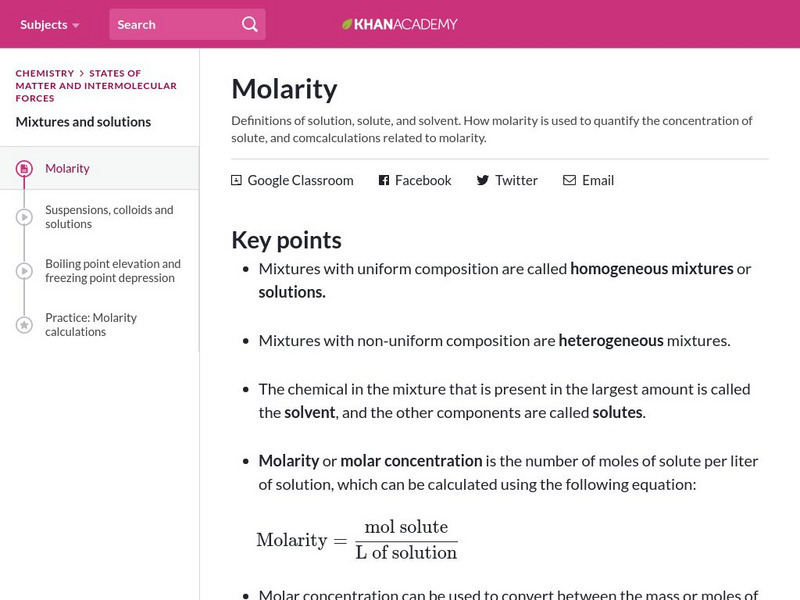
Khan Academy
Khan Academy: Molarity
Learn the definitions of a solution, solute, and solvent. Understand how molarity is used to quantify the concentration of solute, and comcalculations related to molarity.
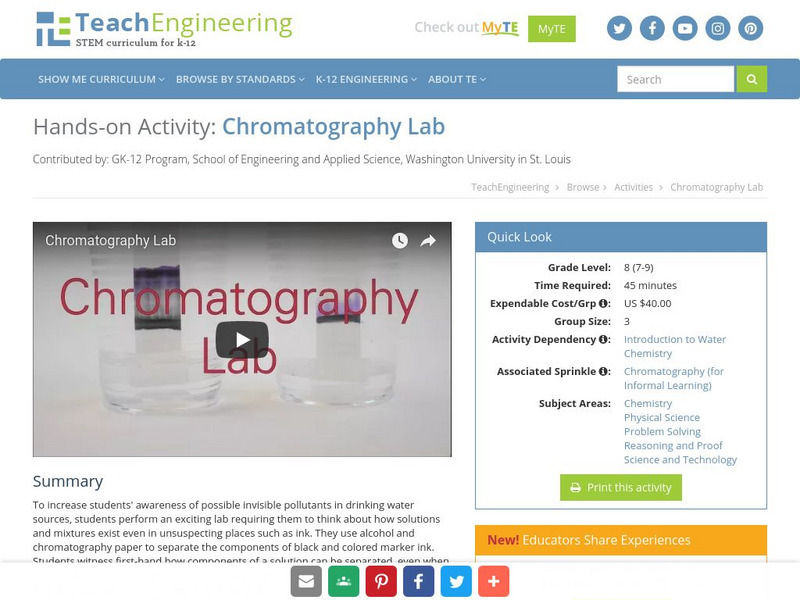
TeachEngineering
Teach Engineering: Chromatography Lab
To increase students' awareness of possible invisible pollutants in drinking water sources, students perform an exciting lab requiring them to think about how solutions and mixtures exist even in unsuspecting places such as ink. They use...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Arrhenius Acid Base Reactions: Audio Book
Listen and learn about Arrhenius acid-base reactions while exploring mixtures, solutions, and their reactions. Examine the photos of the examples discussed in this website and discover how arrhenius acid-base reactions occur.
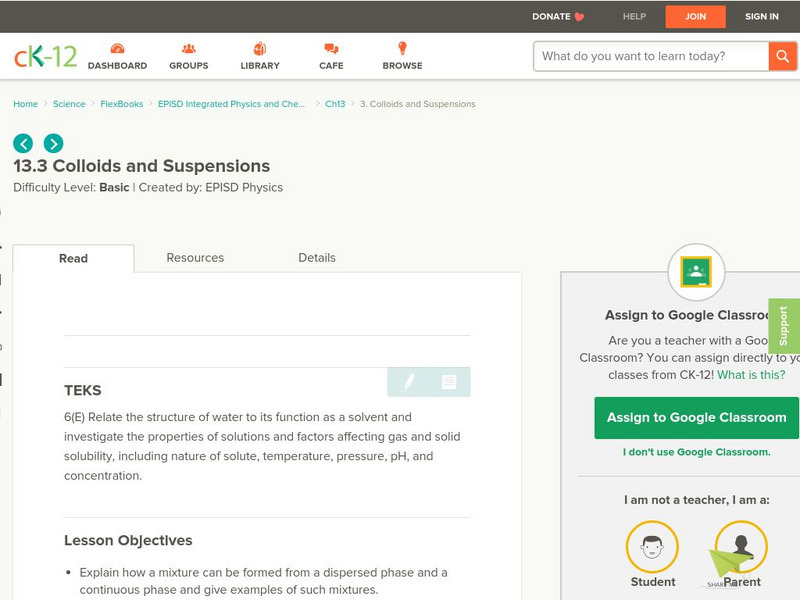
CK-12 Foundation
Ck 12: Colloids and Suspensions
[Free Registration/Login may be required to access all resource tools.] In this lesson, students expand their study of mixtures to show that solids and gases can also act as solvents. Additionally, they take a look at situations in which...
TeachEngineering
Teach Engineering: Concentrate This! Sugar or Salt
Students investigate the property dependence between concentrations and boiling point. First, they investigate the boiling point of various liquid solutions. Then they analyze data collected from the entire class to generate two boiling...
TeachEngineering
Teach Engineering: Concentrate This! Sugar or Salt
Students investigate the property dependence between concentrations and boiling point. In Section 1, students first investigate the boiling point of various liquid solutions. In Section 2 they analyze data collected from the entire class...
Chem Tutor
Wyzant: Lessons: Chemistry: Other Types of Mixtures
A discussion of the "other" mixtures, colloids and suspensions. A good overview of these types of mixtures. It also provides a table of the properties of colloids.















![Chem1 Virtual Textbook: Solutions [Pdf] eBook Chem1 Virtual Textbook: Solutions [Pdf] eBook](https://static.lp.lexp.cloud/images/attachment_defaults/resource/large/FPO-knovation.png)