Hi, what do you want to do?
Other
Science Alive: Chemical Reactions and How You Know When You've Made Something
In this activity, students carry out a chemical reaction in which two reactants (baking soda and hydrochloric acid) produce three products (sodium chloride, carbon dioxide gas, and water) and determine that the solid product (sodium...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Chemical Calculations and Chemical Equations [Pdf]
This chapter on chemical calculations and equations focuses on equation stoichiometry. Learn how to convert masses, use limiting reactants, and calculate the molarity of a solution.
Texas Instruments
Texas Instruments: Conductimetric Titration & Gravimetric Determination
Students use a Conductivity Probe to measure change in conductivity during a chemical reaction and determine the equivalence point of the reaction They determine the mass of the product and calculate the molar concentration of the...
Purdue University
Chem Ed: Chemical Reactions
Because atoms are neither created nor destroyed in a chemical reaction, the total mass of products in a reaction must be the same as the total mass of the reactants.
Chem4kids
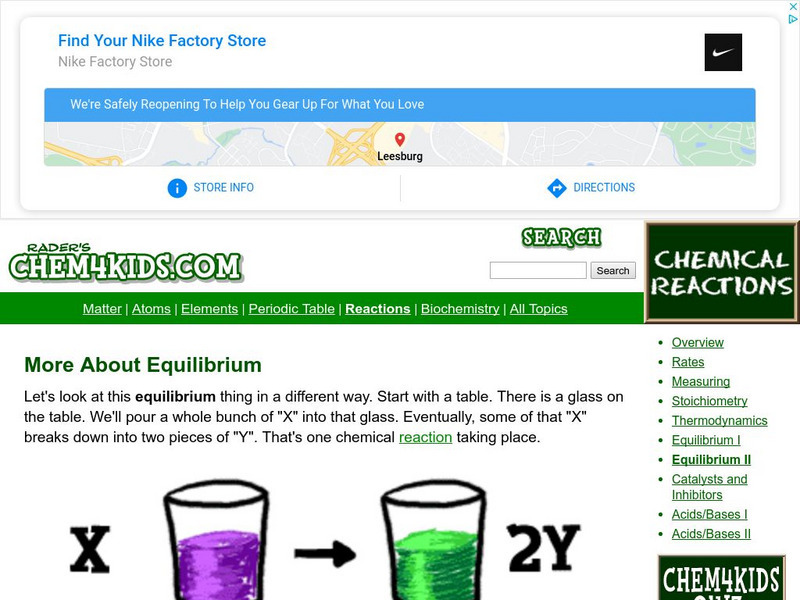
Chem4 Kids: More About Equilibrium
Equilibrium in chemistry is two reactants combining together and then coming apart to their two parts. Review this resource featuring a way to approach chemical equilibrium.
CK-12 Foundation
Ck 12: Stoichiometric Calculations
[Free Registration/Login may be required to access all resource tools.] Based on the balanced chemical equation, students will calculate the masses or moles of reactants or products generated in a given reaction. They will also convert...
TeachEngineering
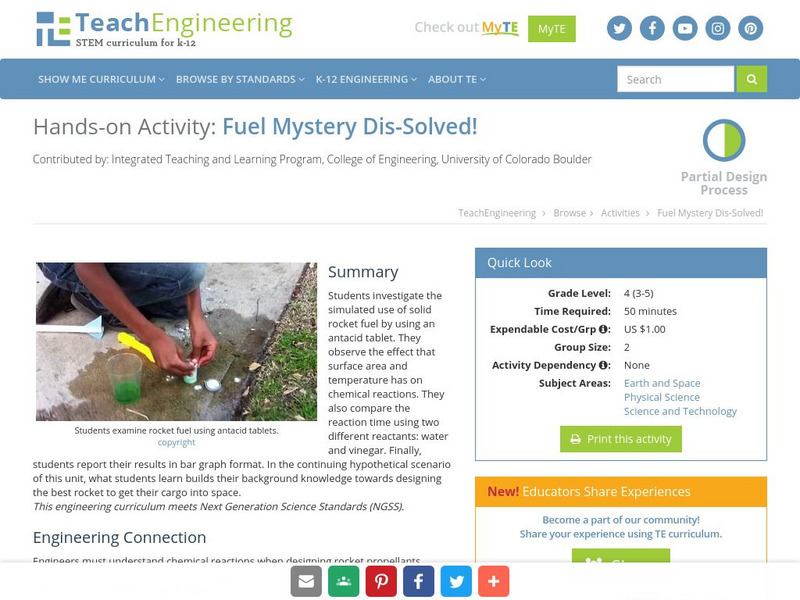
Teach Engineering: Fuel Mystery Dis Solved!
In this activity, students investigate the simulated use of solid rocket fuel by using an antacid tablet. Students observe the effect that surface area and temperature has on chemical reactions. Also, students compare the reaction time...
CK-12 Foundation
Ck 12: Plix: Balancing Chemical Equations
[Free Registration/Login Required] Before you can balance an equation, you need to count the number of each atom present in the reactants and products. Using the example that is shown here, apply the numbers and table to track how many...
BBC
Bbc: Gcse Bitesize: Calculations for All Students
Law of conservation of mass: No atoms are created or destroyed in a chemical reaction. They just join together in a different way than they were before the reaction, and form products. This means that the total mass of the products in a...
CK-12 Foundation
Ck 12: Chemistry Simulation: Flat vs. Fizzy Soda
[Free Registration/Login Required] Explore Le Chatelier's Principle by changing the volume, pressure, and temperature in a bottle of soda. Try carbonating the soda to see how adding a reactant affects the chemical equilibrium.
Utah Education Network
Uen: Hotter All the T Ime
Students will establish the degree of temperature change for a reaction and then change the concentration of one reactant.
Khan Academy
Khan Academy: Stoichiometry
How to use mole ratios from a balanced reaction to calculate amounts of reactants.
Georgia Department of Education
Ga Virtual Learning: Chemistry: Kinetics and Equilibrium
Through informational text, interactive practice problems, virtual simulations, and video clips, students learn about reaction rates and equilibrium.
Chemistry Collective
Chem Collective: Determine the Concentration of Unknown Silver Nitrate Solution
In this limiting reagents problem, students are asked to determine the amount of silver nitrate dissolved in a solution by performing a reaction with solid NaCl. In this randomized activity, each student is given a different unknown...
Chemistry Collective
Chem Collective: Chemical Remediation of Arsenic
In this problem, students determine the limiting reagent in a reaction involving the remediation of arsenic from drinking water.
Chemistry Collective
Chem Collective: Gravimetric Determination of Arsenic
Set in the context of ground water contamination in Bangladesh, this stoichiometry and analytical chemistry activity examines the issues around identifying wells contaminated with arsenic. In this activity, students perform a gravimetric...
Georgia Department of Education
Ga Virtual Learning: Chemistry: Stoichiometry
Through informational text, interactive practice problems, video clips, and real-world application, students are introduced to the science of Stoichiometry.
Utah Education Network
Uen: Chemical Reactions and Equations
With this experiment, students will identify evidence of chemical reactions and demonstrate how chemical equations are used to describe them.
BioMan Biology
Bio Man Biology: Respiration and Photosynthesis Quizzes
A multiple-choice quiz on the processes of respiration and photosynthesis in plants.
Science Education Resource Center at Carleton College
Serc: Investigating Chemical Reactions: Factors Which Influence Rate of Reaction
In this activity, students investigate and discover how surface area, concentration, and temperature affect the rate of a chemical reaction. This lab is designed for students to practice good experimental tests of hypotheses, synthesize...
Center of Science and Industry
Cosi Columbus: Sidewalk Chalk
Make your own sidewalk chalk, and learn about chemical reactions. During the procedure, feel the heat as energy is released in an exothermic reaction.
Concord Consortium
The Concord Consortium: Molecular Workbench: Catalyzed Reactions
Explore the purpose of catalysts in chemical reactions and how they affect the reaction pathways.
CK-12 Foundation
Ck 12: Chemistry: Conservation of Mass
[Free Registration/Login may be required to access all resource tools.] Describes the law of conservation of mass.
CK-12 Foundation
Ck 12: Life Science: Light Reactions of Photosynthesis
[Free Registration/Login may be required to access all resource tools.] Although we generally discuss plants when learning about photosynthesis, keep in mind that plants are not the only organisms that can make their own food. Some...
Other popular searches
- Reactants and Products
- Limiting Reactants
- Chemistry Limiting Reactants
- Reactants of Photosynthesis
- Reactants With Vinegar
- Mass of Reactants
- Excess Reactants
- Introduction to Reactants
- Reactants of Equations