Hi, what do you want to do?
Sophia Learning
Sophia: Chemical Reactions: Stoichiometry
This lesson on stoichiometry demonstrates how to use a balanced chemical equation to determine the mass of a product given the starting mass of a reactant. [7:55]
Other
Nearpod: Photosynthesis
In this lesson on exploring photosynthesis, students will explore photosynthesis by examining the reactants and products of this process.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Real World Applications of Equation Stoichiometry
This audio book, narrated by author Mark Bishop, describes how equation stoichiometry can be used in the real world. Many examples are given to help understand how to plan chemical reactions, solve for limiting reactants, and calculate...
BBC
Bbc: Gcse Bitesize: Chemical Calculations
You should be able to calculate the masses of reactants and products from balanced equations, and the formula of a compound from information about reacting masses.
CK-12 Foundation
Ck 12: Plix: Balancing Chemical Reactions
[Free Registration/Login Required] Demonstrate a normal chemical reaction by dragging atoms from the products to create the reactants in this chemical equation.
Khan Academy
Khan Academy: Limiting Reagents and Percent Yield
How to determine the limiting reagent, and using stoichiometry to calculate the theoretical and percent yield.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Equilibrium Pressure of Reactants and Products
Learn about the equilibrium pressures of chemical reactions. Follow the general steps used in calculating the pressures, and see some examples. Also find links to tutorials, presentation, and animations about other chemistry topics.
Frostburg State University
Frostburg State University: Limiting Reagents
Middle of a slide show on stoichiometry that shows a nice graphical look and example of limiting reagents.
Shodor Education Foundation
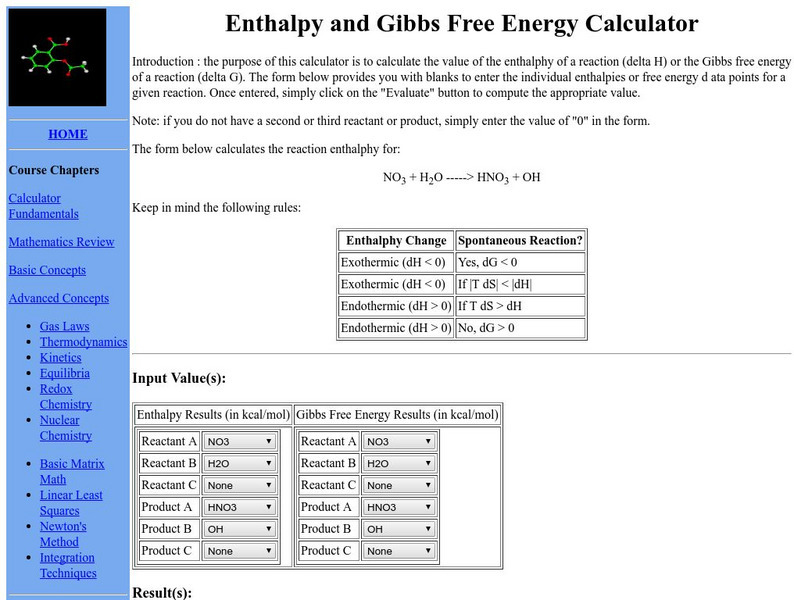
Shodor: Enthalpy and Gibbs Free Energy Calculator
Interactive calculator that allows you to input reactants and products of a reaction and calculate the change in enthalpy and Gibbs Free Energy for the reaction.
Dartmouth College
Dartmouth College: Chem Lab: Titration
A titration is a method of analysis that will allow you to determine the precise endpoint of a reaction and therefore the precise quantity of reactant in the titration flask. This site demonstrates how to correctly perform this analysis.
CK-12 Foundation
Ck 12: Types of Chemical Reactions
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will define and give general equations for combination, decomposition, single-replacement, and double-replacement...
CK-12 Foundation
Ck 12: Chemical Equations
[Free Registration/Login may be required to access all resource tools.] Students explore mass relations between reactants and products for a given chemical process, and practice balancing chemical equations.
CK-12 Foundation
Ck 12: Types of Chemical Reactions
[Free Registration/Login may be required to access all resource tools.] In this learning module, studnets will classify a chemical reaction as a combination, decomposition, single replacement, double replacement, or combustion reaction....
Khan Academy
Khan Academy: The Equilibrium Constant K
Reversible reactions, equilibrium, and the equilibrium constant K. Learn how to calculate K, and how to use K to determine if a reaction strongly favors products or reactants at equilibrium.
TeachEngineering
Teach Engineering: Reaction Exposed: The Big Chill!
Students investigate the endothermic reaction involving citric acid, sodium bicarbonate and water to produce carbon dioxide, water and sodium citrate. In the presence of water [H2O]; citric acid [C6H8O7] and sodium bicarbonate [NaHCO3]...
Chemistry Collective
Chem Collective: Camping Problem Ii
In this part of the MRE scenario, students determine change in the enthalpy of a reaction as the concentration of reactants are varied.
CK-12 Foundation
Ck 12: Connecting Cellular Respiration and Photosynthesis
[Free Registration/Login may be required to access all resource tools.] How do trees help you breathe? This activity covers the connection between cellular respiration and photosynthesis and identifies the products and reactants of each.
American Chemical Society
Middle School Chemistry: Lesson Plans: Temperature and Rate of Chemical Reaction
Students experiment to see if the temperature of two clear colorless solutions affects how fast they react.
Other
Chemical Reax/exothermic Reactions
A wonderfully complete primer on exothermic reactions in the context of chemical spills. Covers Reactions with Air or Water, Combustible Organics, Polymerization Reax, Decomposition, Corrosion, and much much more.
Chem4kids
Chem4 Kids: Stoichiometry
This site provides a great overview of stoichiometry, the part of chemistry that studies amounts of substances that are involved in reactions. Content focuses on what you measure, and includes two examples.
Chemistry Collective
Chem Collective: Stoichiometry and Solution Preparation Problem
In this limiting reagents problem, students mix together solutions in different ratios in an attempt to produce a final solution that contains only 1 product.
Chemistry Collective
Chem Collective: Unknown Concentration of Dna Solution Problem
In this advanced limiting reagent problem, students use the virtual lab to determine the concentration of a solution of DNA by reacting it with known amounts of a fluorescent dye which binds to the DNA.
OpenStax
Open Stax: Chemical Reactions
This site provides an overview of the role of energy in chemical reactions.
American Chemical Society
Middle School Chemistry: Controlling Amount of Products in a Chemical Reaction
Students analyze the chemical equation for the reaction between vinegar and baking soda. They observe that the gas produced in the reaction is also part of the products of the written chemical equation.
Other popular searches
- Reactants and Products
- Limiting Reactants
- Chemistry Limiting Reactants
- Reactants of Photosynthesis
- Reactants With Vinegar
- Mass of Reactants
- Excess Reactants
- Introduction to Reactants
- Reactants of Equations