Hi, what do you want to do?
CK-12 Foundation
Ck 12: Physical Science: Elements
[Free Registration/Login may be required to access all resource tools.] Discusses elements and their different properties, the history of elements, and how atoms relate to elements.
CK-12 Foundation
Ck 12: Physical Science: Elements
[Free Registration/Login may be required to access all resource tools.] Explores elements and their different properties, the history of elements, and how atoms relate to elements.
CK-12 Foundation
Ck 12: Fifth Grade Science: Physical Science: Types of Matter
[Free Registration/Login may be required to access all resource tools.] Discusses elements, atoms, compounds, molecules, and crystals. Looks at mixture and different types of them.
CK-12 Foundation
Ck 12: Physical Science: Valence Electrons
[Free Registration/Login may be required to access all resource tools.] Valence electrons, their variation in the periodic table and relation to reactivity and electrical conductivity of elements.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Spin
A basic explanation of spin and how it relates to electrons and quantum numbers.
Museum of Science
The Atom's Family: Phases of Matter
Help the Phantom choose a material and observe the changes at different temperatures in the molecule chamber. What happens to the elements or molecules as the temperature changes?
Wikimedia
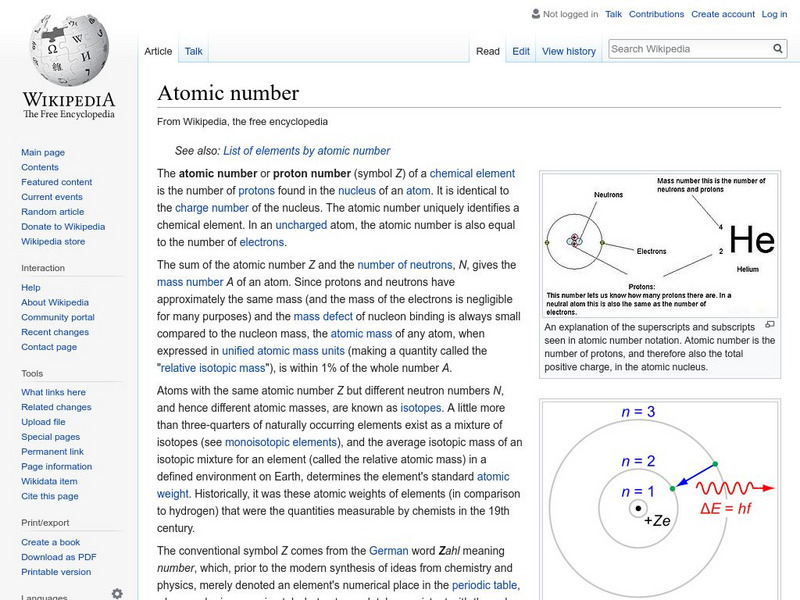
Wikipedia: Atomic Number
Wikipedia provides the definition of the term, "Atomic number," a term used in chemistry and physics to represent the number of protons in the nucleus of an atom.
National High Magnetic Field Laboratory
Magnet Academy: Enrico Fermi
Enrico Fermi was a titan of twentieth-century physics. He outlined the statistical laws that govern the behavior of particles that abide by the Pauli exclusion principle and developed a theoretical model of the atom in his mid-twenties....