Environmental Chemistry
Environmental chemistry.com: Anatomy of an Atom
Explains the basics of atomic structure, from simple definitions to information about quantum theory. Accurate and helpful basics whether or not you need the more advanced information.
NASA
Nasa: Atoms and Molecules in Motion, States of Matter [Pdf]
Everythingin the universeis either matter or energy. All matter is made of combinations of about one hundred basic building blocks, the chemical elements on the periodic table. Going a step further and breaking these elements down to...
Department of Defense
Do Dea: Chemistry Review
Review atoms with this slideshow. You can choose to listen to the review or read the accompanying text. At the end, play the game to review the basics of atoms that you have just learned about. You may play the game as many times as...
CK-12 Foundation
Ck 12: Plix: Build Some Helium: Atoms to Molecules
[Free Registration/Login Required] Build your own helium atom and make sure it has the correct number of protons, electrons, and neutrons on this site. Site also includes a small quiz on the topic.
Tom Richey
Slide Share: Atomic Structure
Slideshow looking at the history of models of the atom, including those proposed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Discusses subatomic particles, including the numbers of protons, neutrons,...
Science Education Resource Center at Carleton College
Serc: Drawing Atoms
This activity serves as an introduction to chemistry, and can be used to help students draw a two dimensional image of the atom.
Thomas Jefferson National Accelerator Facility
Jefferson Lab: It's Elemental: Element Math Game
The interactive activity examines the Periodic Table of Elements. Learners answer questions about the number of protons, electrons, neutrons, or nucleons that an atom of an element contains.
Other
Scientists Say Star Collision Caused Dinosaur Extinction
"Israeli scientists have a new theory on why the dinosaurs became extinct: cosmic radiation that bombarded the Earth following the collision of two neutron stars." This article discusses the details of the theory.
Thomas Jefferson National Accelerator Facility
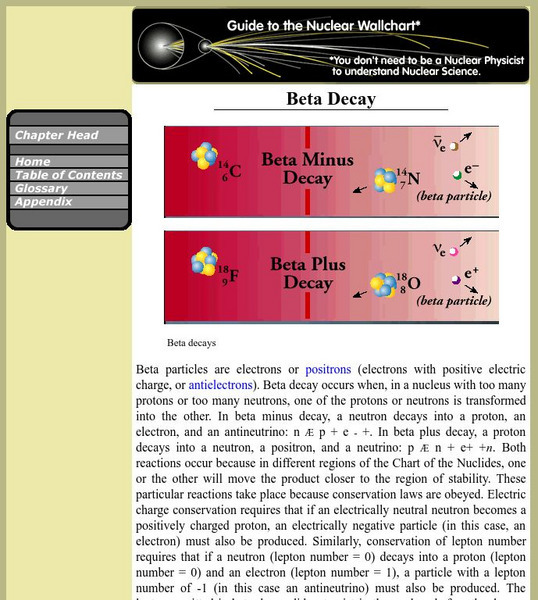
Jefferson Lab: Beta Decay
This site from Jefferson Lab provides a description of beta decay along with two helpful formula examples. Several links are provided throughout this page for additional information on related subjects.
Sophia Learning
Sophia: Subatomic Particles: The Proton: Lesson 2
This lesson will explain that protons are positively charged particles having a mass of one amu and are found in the nucleus of the atom. It is 2 of 3 in the series titled "Subatomic Particles: The Proton."
Lawrence Berkeley National Laboratory
Berkeley Lab: Beta Decay
Entry explores the process of beta decay which occurs when, in a nucleus with too many protons or too many neutrons, one of the protons or neutrons is transformed into the other.
ClassFlow
Class Flow: Element Math
[Free Registration/Login Required] In this flipchart students determine the number of protons, neutrons, and electrons of elements essential to life. They use Activotes to check knowledge gained. Students create a Bohr Model.
Mocomi & Anibrain Digital Technologies
Mocomi: Structure of an Atom
An atom is made of three parts - protons, neutrons, and electrons. Here you can explore these different parts.
Thomas Jefferson National Accelerator Facility
Jefferson Lab: All About Atoms
The three basic particles that make up atoms are defined and illustrated.
Sophia Learning
Sophia: Atomic Number: Lesson 3
This lesson explains what is represented by the atomic number, and how it varies from one element to the next. It is 3 of 7 in the series titled "Atomic Number."
Sophia Learning
Sophia: Atomic Number: Lesson 6
This lesson explains what is represented by the atomic number, and how it varies from one element to the next. It is 6 of 7 in the series titled "Atomic Number."
Sophia Learning
Sophia: Atomic Number: Lesson 5
This lesson explains what is represented by the atomic number, and how it varies from one element to the next. It is 5 of 7 in the series titled "Atomic Number."
Science Struck
Science Struck: Bromine Isotopes
Describes the chemical properties of bromine and what an isotope is. Presents a chart of bromine isotopes listing nuclide symbol, neutron number, isotopic mass, and half-life, as well as a second simpler chart with more bromine isotopes.
Concord Consortium
Concord Consortium: How Does an Object Become Charged?
Activity 1 in this module: What is the effect of changing the composition of an atom? Since all atoms contain protons, neutrons, and electrons, what makes one element different from another is examined.
Curated OER
[Bohr Model of Unununium]
A look at the transition metal unununium, which was renamed roentgenium in 2004. Includes basic information, including symbol, number, mass, melting and boiling points, and numbers of protons, electron, and neutrons.