Hi, what do you want to do?
Sophia Learning
Sophia: Mixed Conversion Problems: Lesson 2
This lesson explains how to approach practice problems when trying to determine what final quantity is being asked for. It is 2 of 2 in the series titled "Mixed Conversion Problems."
Indiana University
Indiana University Northwest: Molar Mass
This site shows how to compute molar mass by working through an example.
Other
Widener University: Ideal Gas Law
At this site from the Widener University Department of Chemistry, you can try your skill with using the ideal gas law equation to solve problems. This page contains an interactive JavaScript form which delivers problem after problem. You...
CK-12 Foundation
Ck 12: Stoichiometric Calculations
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will learn to calculate the amount in moles of a reactant or product from the mass of another reactant or product. They...
CK-12 Foundation
Ck 12: Limiting Reactant and Percent Yield
[Free Registration/Login may be required to access all resource tools.] Students will compare theoretical yield to actual yield, and then investigate what happens when one reactant runs out before the other reactants are fully consumed....
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Molarity
What determines the concentration of a solution? Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Change solutes to compare different chemical compounds in water.
CK-12 Foundation
Ck 12: Gas Properties
[Free Registration/Login may be required to access all resource tools.] The following online tutorial describes how a gas can be compressed and identifies three factors that affect gas pressure. Students will describe the effects...
Frostburg State University
General Chemistry Online: Limiting Reactant Problem
Explains the essence of limiting reactant problems using a sample problem: How can an amount of product (KNO3) be predicted from amounts for two reactants (KCl and HNO3)? Shows the work and explains how to solve these types of problems.
Chem4kids
Chem4 Kids: Symbols in Chemical Equations
Chem4Kids! provides an overview of the symbols representing numerical values in chemical equations. Each symbol is defined and described.
Other
Bipm: The International System of Units (Si)
BIPM (Bureau International des Poids et Mesures) is an international organization, located in France, an equivalent of our National Institute of Standards and Technology (NIST). This site is the BIPM intro page to their official...
Royal Society of Chemistry
Royal Society of Chemistry: Gridlocks: Level 3
A collection of grid puzzles that cover a wide variety of topics in advanced high school chemistry. These are excellent for topic review and reinforcement. The puzzles can be played online and also downloaded as worksheets. Answers are...
Royal Society of Chemistry
Royal Society of Chemistry: Gridlocks: Level 2
A collection of grid puzzles that cover a wide variety of topics in intermediate high school chemistry. These are excellent for topic review and reinforcement. The puzzles can be played online and also downloaded as worksheets. Answers...
CK-12 Foundation
Ck 12: The Ideal Gas Law
[Free Registration/Login may be required to access all resource tools.] Students read about and explore video clips, diagrams, and example problems using the Ideal Gas Law.
CK-12 Foundation
Ck 12: Solubility Equilibrium
[Free Registration/Login may be required to access all resource tools.] Students write the solubility product constant expressions for nearly insoluble ionic compounds and perform calculations for compounds and solubility.
CK-12 Foundation
Ck 12: Flex Book Textbooks: Chemistry Second Edition
[Free Registration/Login may be required to access all resource tools.] A complete, web-based, multi-media textbook covering a wide variety of Chemistry concepts.
Smithsonian Institution
Smithsonian National Zoo: Small Mammals
The Smithsonian National Zoological Park explores the lives of small mammals, both in the wild and in captivity. Choose a webcam to view a variety of small mammals live and up-close, learn about current conservation efforts, access an...
Smithsonian Institution
Smithsonian National Zoo: Animal Cams
This site features a variety of live animal cams including cams of the lions, the giant pandas, and the elephants. In addition to the live cams, information on each animal is provided.
Iowa State University
Iowa State University: Iowa Insect Information Notes Crickets
Crickets can be annoying when they get into a house. These information sheets provide ways to prevent crickets from becoming a pest problem while also explaining some basic cricket biology.
Chemistry Collective
Chem Collective: Creating a Stock Solution
In this activity, students use the virtual lab to create dilute solutions from a concentrated stock solution of acids or bases. They must first calculate the correct volumes of concentrated acid solution and water to mix together to...
Chemistry Collective
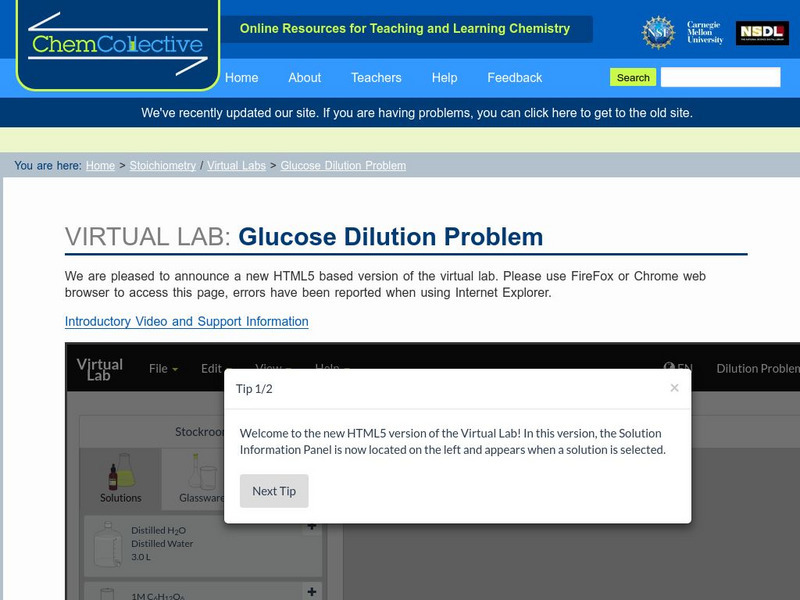
Chem Collective: Glucose Dilution Problem
In this activity, students use the virtual lab to create a 0.025M glucose solution from a standard 1M glucose solution. First, they calculate the correct volumes of 1M glucose solution and water to mix together to create the final 0.025M...
Chemistry Collective
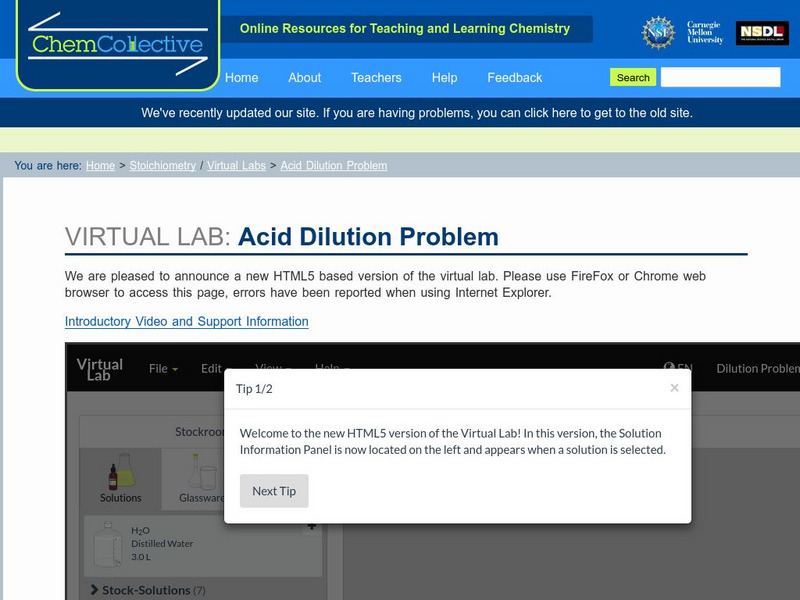
Chem Collective: Acid Dilution Problem
In this activity, students use the virtual lab to create 500mL of 3M HCl solution from a concentrated stock solution of 11.6M HCl. They must first calculate the correct volumes of 11.6M HCl solution and water to mix together to create...
Chemistry Collective
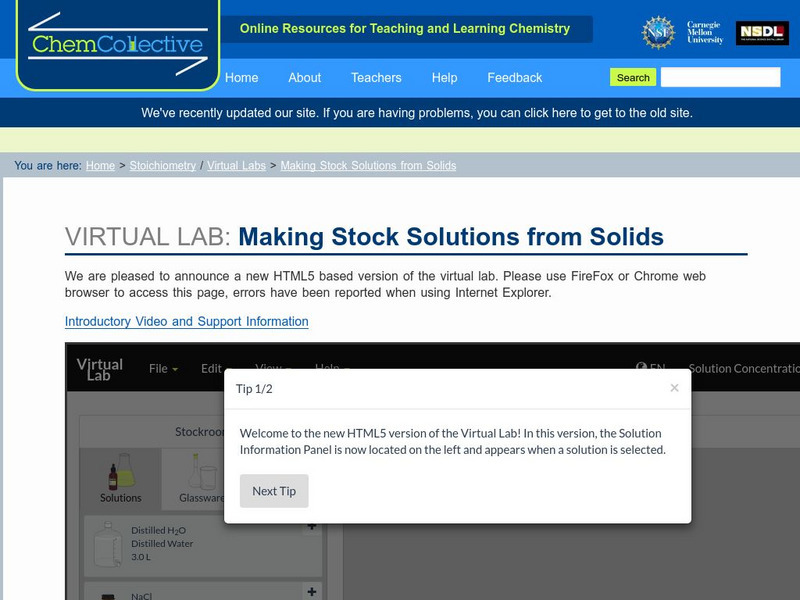
Chem Collective: Making Stock Solutions From Solids
In this activity, students use the virtual lab to create stock solutions starting from solid salts. Students must first calculate the correct amount of solid to make the solution. Next, they prepare the solution using the appropriate...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Gases and Their Properties: Audio Book
This audio book discusses the properties of gases. The author talks about many gas relationships: pressure/volume, pressure/temperature, volume/temperature, pressure/moles, and volume/moles.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Molar Mass Conversion Factors
Test your knowledge of moles by practicing this animated exercise. Fill in the conversion factors for the molar mass and see if you are a mole master!
Other popular searches
- Molecules
- Molecular Biology
- Atoms and Molecules
- Mole Concept
- Molecular Geometry
- Gumdrop Atoms and Molecules
- Atoms & Molecules
- Compounds and Molecules
- Water Molecules
- Organic Molecules
- Kinetic Molecular Theory
- Atoms Molecules