Hi, what do you want to do?
University Corporation for Atmospheric Research
Ucar: Just a Phase: Water as a Solid, Liquid, and Gas
This site helps students construct a model of the arrangement of water molecules when present as solid, liquid or gas. Includes background information, lesson plans, links to standards and assessment ideas.
Museum of Science
The Atom's Family: Mighty Molecules
In this activity, students construct models of molecules using marshmallows and gum drops.
American Chemical Society
Middle School Chemistry: Chapter 5: The Water Molecule and Dissolving
Nine exemplary chemistry lessons about the water molecule and dissolving complete with handouts and animations.
State University of New York
State University of New York: Kinetic Molecular Theory
This module simulates the behavior of a small number of gas molecules in a small box.
Texas Education Agency
Texas Gateway: Kinetic Theory: Atomic & Molecular Explanation of Pressure & Temp
By the end of this section, you will be able to express the ideal gas law in terms of molecular mass and velocity; define thermal energy; calculate the kinetic energy of a gas molecule, given its temperature; describe the relationship...
Simon Fraser University
Chem1 Virtual Textbook: Molecules in Motion
As part of the General Chemistry Virtual Textbook, this site continues a study conducted on various topics related to gases. As a specific topic, the site covers an introduction to kinetic molecular theory.
Other
Beautiful Chemistry: Beautiful Structures: Gas and Liquid
In Beautiful Chemistry, we talk about crystals, quasicrystals and glasses, which are all solids. Here, we see the molecular structure of gases and liquids.
University of Florida
Chem. 2041 Lecture Notes: The Forces Between Molecules
A discussion of the variety of forces which hold molecules together. The relative strengths of these forces for the various states of matter is discussed. The effect of such forces on the boiling points and other phase change...
OpenSciEd
Open Sci Ed: Net Logo: Gas Particle Collisions Up Close
This simulation explores the relationship between particle kinetic energies during particle collisions.
Concord Consortium
Concord Consortium: Gas Pressure in a Syringe
Explore why it is difficult to pull back on a capped syringe and why the plunger moves back in when you let go.
Simon Fraser University
Chem1 Virtual Textbook: Kinetic Molecular Model
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with the basic components of the Kinetic-Molecular Theory in addition to...
Other
Virtual Chembook: Density Applications With Gases
DENSITY is a physical property of matter, as each element and compound has a unique density associated with it. Density defined in a qualitative manner as the measure of the relative "heaviness" of objects with a constant volume. For...
University Corporation for Atmospheric Research
Ucar: Greenhouse Gases Sorting Game
Sort these molecules as greenhouse gases, borderline cases or not greenhouse gases.
New York University
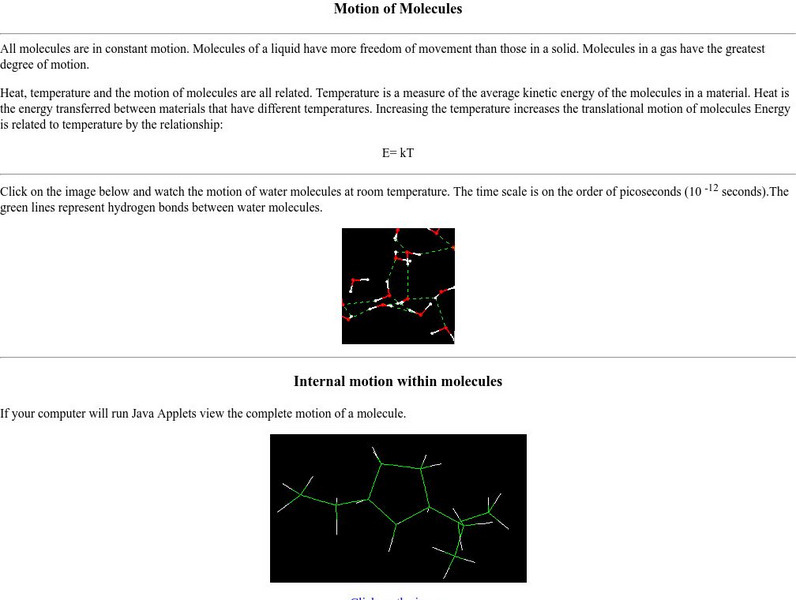
Nyu: Math Mol: Motion of Molecules
Examine the link between molecular motion and energy. Observe the movement of a molecule at room temperature. Learn about the different types of molecular motion.
CK-12 Foundation
Ck 12: Plix Series: Gas Mixture and Molecular Speeds
[Free Registration/Login Required] Hike to the top of Mt. Everest and observe what happens to the amount of molecules pushing down on the surface of the Earth. After the activity, answer one challenge question about the topic.
Simon Fraser University
Chem1 Virtual Textbook: Light Bulbs
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with light bulbs and their relation to information on movement of gases and...
Simon Fraser University
Chem1 Virtual Textbook: Diffusion: Random Motion With Direction
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with diffusion along with topics such as concentration gradient and random...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: States of Matter
Watch different types of molecules form a solid, liquid, or gas. Add or remove heat and watch the phase change. Change the temperature or volume of a container and see a pressure-temperature diagram respond in real time. Relate the...
Vision Learning
Vision Learning: States of Matter
Did you know the ancient Greeks first identified the three states of matter? Read about how the molecules differ in solids, liquids, gases, and plasma. View photographs and watch how the molecules move in each state. If you're really...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: The Greenhouse Effect
How do greenhouse gases affect the climate? Explore the atmosphere during the ice age and today. What happens when you add clouds? Change the greenhouse gas concentration and see how the temperature changes. Then compare to the effect of...
Texas Education Agency
Texas Gateway: Kinetic Molecular Theory
[Accessible by TX Educators. Free Registration/Login Required] This resources allows students to explore the postulates of the Kinetic Molecular Theory in order to better understand why gas particles behave the way that they do.
Khan Academy
Khan Academy: What Is the Maxwell Boltzmann Distribution?
In a gas, there are lots of molecules traveling at lots of different speeds. Here's a framework for thinking about that.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: States of Matter: Basics
After watching this simulation, students will be able to describe characteristics of the three states of matter. They will watch as atoms and molecules change between solid, liquid and gas phases.
Other
The Science House: Floating Candles
In this experiment students observe a combustion reaction and deduce the components necessary for the reaction to occur. They also see the relationship between pressure, volume, and number of molecules for gases.