Science Education Resource Center at Carleton College
Serc: Valence Electrons and Trends in the Periodic Table
This instructor led activity will produce a partially filled periodic table that contains electron-dot models for the first twenty elements in the appropriate boxes. It will be used as a visual tool for students to connect concepts such...
Simon Fraser University
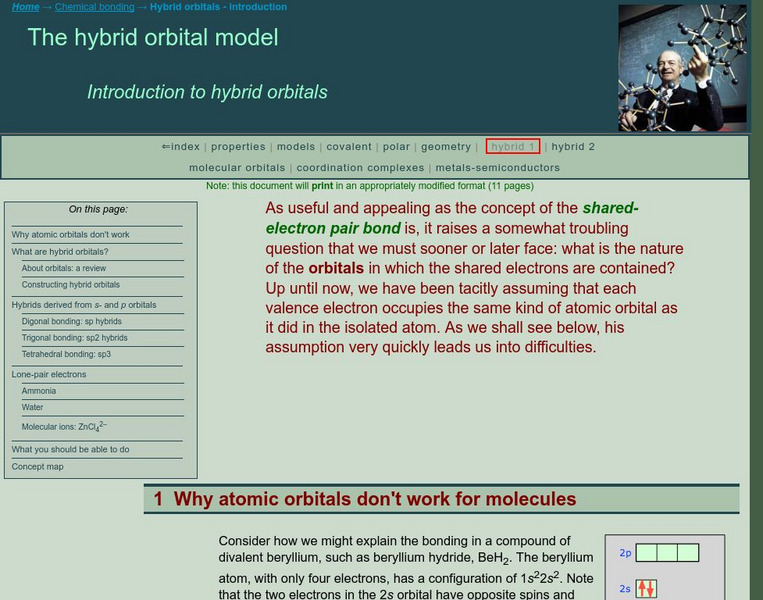
Chem1 Virtual Textbook: The Valence Bond Model
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with hybrid orbitals and specifically the valence bond model.
Simon Fraser University
Chem1 Virtual Textbook: Models of Chemical Bonding: Why Do Atoms Join Together?
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with chemical bonds and seeks to answer the question, why do chemical bonds form?
Quia
Quia: Chemical Bonds (Ionic and Covalent) Quiz
This is 21-question multiple choice quiz over chemical bonds was written for a 7th-grade science class.
Other
Us: Valence Shell Electron Pair Repulsion (Vsepr)
An excellent tutorial that examines VSEPR and pairs of valence electrons. The valence shell electron pair repulsion concept is explored using animated models. Includes a VSEPR calculator. Use the toolbar on the left to navigate through...
State University of New York
State University of New York: Atomic Electron Configuration
This simulation displays the electron configurations of for the elements in relation to the element's position on the periodic table.
State University of New York
State University of New York: Electron Configurations
The following simulation provides an interaction with electron configurations.
Davidson College
Davidson College: Valence Shell Electron Pair Repulsion (Vsepr) Model
Presents examples of molecules that do not match the expected bond angles in the Valence-Shell Electron-Pair Repulsion Model. Requires Java.
Other
Chemical Bonds: Formal Charges
A slide presentation with several slides dedicated to the topic of formal charges. Formal charges are explained, and examples are given. A practice example is provided.
Simon Fraser University
Chem1 Virtual Textbook: Electron Tunneling Model
Find out about shared electrons between the two nuclei. Learn about uncertainty at work.
Sophia Learning
Sophia: Metallic Bonding
A video tutorial helps illustrate a metallic bond, and explains several metallic properties based on the arrangement of electrons. [3:22]
Wisc-Online
Wisc Online: Lewis Dot Structures of Covalent Compounds
Short slide show provides basic information about drawing Lewis dot structures for covalent compounds. Starts with anatomy of the atom, and then shows the relationship between atomic particles and the Periodic Table of Elements. Offers...
Sophia Learning
Sophia: Valence Electrons: Lesson 6
This lesson will describe the importance of valence electrons in the process of chemical bonding. It is 6 of 7 in the series titled "Valence Electrons."
Sophia Learning
Sophia: Characteristics of Chemical Bonds: Lesson 2
This lesson will present the basic properties and characteristics of chemical bonds. It is 2 of 4 in the series titled "Characteristics of Chemical Bonds."
Sophia Learning
Sophia: Characteristics of Chemical Bonds: Lesson 4
This lesson will present the basic properties and characteristics of chemical bonds. It is 4 of 4 in the series titled "Characteristics of Chemical Bonds."
Frostburg State University
General Chemistry Online: What Is a Valence Bond?
Brief explanation from Frostburg State University of valence bond theory and an explanation of what chemical situation creates a valence bond between two atoms.
Michael Blaber, PhD
Fsu: Basic Concepts of Chemical Bonding: Exceptions to the Octet Rule
Lists the exceptions to the octet rule and provides a discussion and diagrams explaining each one. Includes clear diagrams illustrating this concept.
Utah Education Network
Uen: Grouping Bonds
Students will group a selection of electron dot structures and determine similarities and differences between covalent and ionic bonding.
Crescent Public Schools
The Internet Science Room: Oxidation Numbers and Chemical Bonding
Through animated diagrams and illustrated examples, students can further their understanding of chemical bonds and oxidation numbers.
Michael Blaber, PhD
Florida State University: Molecular Geometry and Bonding Theories
This article from the Florida State University contains information on multiple bonds and orbitals. Great charts and pictures are shown to help the educational process. Definitely a great site to check out on the subject.
CK-12 Foundation
Ck 12: Plix Series: Resonance: Metallic Bonding
[Free Registration/Login Required] Manipulate the electrons and protons to explore metallic bonding. Then answer a self-correcting concept question.
Other
Web Chem: Bonding: Valence and Core Electrons
This site from WebChem contains information on the subject of bonding with an emphasis on valence and core electrons.
McMaster University
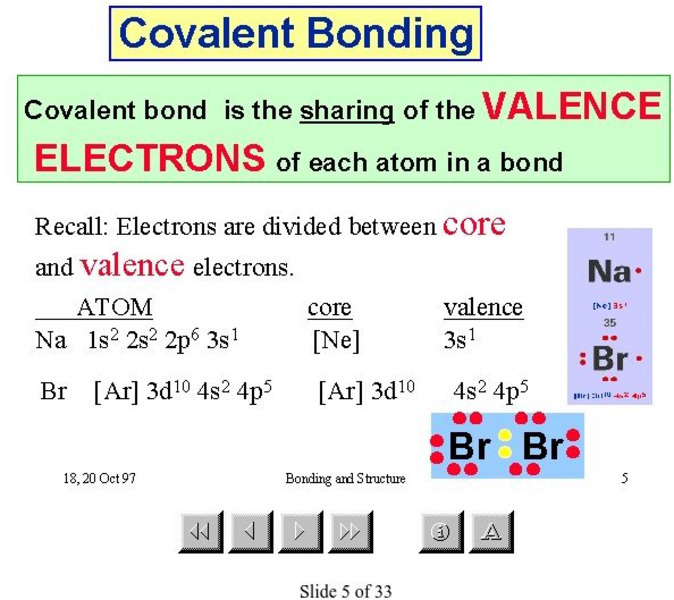
Mc Master University: Covalent Bonding
Slides 5 through 8 in this presentation from the McMaster University explain covalent bonding.
BBC
Bbc: Gcse Bitesize: Covalent Bonds
A covalent bond is formed between non metal atoms, which combine together by sharing electrons. Covalent compounds have no free electrons and no ions so they don't conduct electricity.