TED-Ed
The life cycle of a cup of coffee | A.J. Jacobs
How many people does it take to make a cup of coffee? For many of us, all it takes is a short walk and a quick pour. But this simple staple is the result of a globe-spanning process whose cost and complexity are far greater than you...
Curated Video
Electrolysis of Aqueous Copper Sulfates
The video is a lecture presentation on the electrolysis of aqueous copper sulfates. It begins with an explanation of what electrolysis is and what is in the aqueous solution of the metal salt. The presenter then goes on to make some...
Curated Video
GCSE Chemistry - Electrolysis Part 3 - Aqueous Solutions #42
In this video we cover: - How the electrolysis of soluble compounds works - The rules to find out which ions are discharge at each electrode - Electrolysis of aqueous copper sulfate - Electrolysis of aqueous sodium chloride This video is...
Curated Video
Electrolysis of Aqueous Solutions
This video is a lecture presentation on the topic of electrolysis of aqueous solutions. It starts with a brief refresher on what electrolysis is, and then explains how electrolysis is used to split compounds using electricity. The...
Curated Video
Electrolysis of Ionic Compounds: Molten and Aqueous Solutions
In this video, the process of electrolysis is discussed, which involves the splitting of an ionic compound into its constituent elements by passing an electric current through it. The video explains how electrolysis works for molten lead...
Curated Video
Electrolysis of Aqueous Copper Sulfate Experiment
This is a lecture presentation on the electrolysis of aqueous copper sulfate. The presenter goes through the theory of the electrolysis with metal electrodes and develops a hypothesis about what happens when copper sulfate solution is...
Curated Video
Methods of Extracting Copper from Low Grade Ores: Bioleaching and Phytomining
The video explains two methods of extracting copper from low-grade copper ores. The first method is called bioleaching, which uses bacteria to produce a leachate solution containing copper ions that are then purified using electrolysis....
Mister Simplify
Mayo's Hawthorne Effect (Employee Motivation) - Simplest Explanation ever
Elton Mayo's Hawthorne Effect is a motivational theory used in business to understand the driving factors to employee motivation and retention. This video continues the theme of motivational theories we have been covering. It is a...
Curated Video
Electrolysis of Aqueous Copper Sulfates: Analysis and Conclusion
The video is a lecture presentation on the electrolysis of aqueous copper sulfates. The presenter discusses an experiment to test the hypothesis about the relationship between the current through the electrolyte and the mass of copper...
Curated Video
Electrolysis of Aqueous Solutions: Theory and Applications
This is a video discussing the theory and practical applications of electrolysis of aqueous solutions. The speaker explains what electrolysis is and how it involves the splitting of compounds using electricity. They then dive into the...
FuseSchool
What Is Electrolysis
Electrolysis is electrical current flow through a liquid which causes chemical changes. The liquid can be a molten ionic compound or aqueous solution. The liquid will contain free-flowing positive ions and negative ions. The positive...
Curated Video
GCSE Chemistry - Electrolysis Part 1 - Basics and Molten Compounds #40
This video covers: - What electrolysis is - The equipment of electrolysis and how it should be set up - An example of electrolysis with a molten compound
FuseSchool
Purifying Copper
Learn the basics about Purifying copper. What methods and techniques are used in purifying copper? Find out more in this video!
Curated Video
Metal Extraction
We extract copper metal from copper chloride solution using electrolysis. The electric current causes copper to form at one electrode and chlorine gas to form at the other. The presence of chlorine is tested using blue litmus paper....
Curated Video
Ion Migration
We separate the ions in copper dichromate gel using electrolysis. We see the positive blue copper ions move to the negative electrode and the negative yellow chromate ions move to the positive electrode. Chemistry - Reactions - Learning...
FuseSchool
What Are Half Equations
In this video, we will learn how to write half equations for simple redox reactions. A half-equation shows what happens at one of the electrodes during electrolysis, so we need to be able to complete and balance half-equations for these...
Curated Video
Reactivity Series: Reactive Metals
The discovery and uses of reactive metals throughout history, and how their uses have defined the period in which they were discovered. Chemistry - Periodic Table - Learning Points. The reactivity series ranks the chemical reactivity of...
Brian McLogan
How to multiply two two digit whole numbers together
👉 You will learn how to multiply integers from one digit to multiple digits. When multiplying it is important to understand that multiplication is just repeated addition. However with multi-digit numbers we will follow a step by step...
FuseSchool
How Does Electroplating Work
Learn the basics about electroplating. The anode is positively charged, and the cathode is negatively charged. They are immersed in a solution called an electrolyte. The electrolyte and the anode are selected based upon the material that...
Crash Course
Electrochemistry: Crash Course Chemistry
Chemistry raised to the power of AWESOME! That's what Hank is talking about today with Electrochemistry. Contained within, Hank discusses electrochemical reactions, half reactions, how batteries work, galvanic cells, voltage, standard...
msvgo
Electrochemical Principles of Metallurgy
It explains the extraction of metals by electrolysis.
Curated Video
Peru, Ollantaytambo archaeological site
Around the mid-15th century, the Inca emperor Pachacuti conquered and razed Ollantaytambo; the town and the nearby region were incorporated into his personal estate.The emperor rebuilt the town with sumptuous constructions and undertook...
Curated Video
What Are Half Equations | Reactions | Chemistry | FuseSchool
In this video, we will learn how to write half equations for simple redox reactions. A half-equation shows what happens at one of the electrodes during electrolysis, so we need to be able to complete and balance half-equations for these...
Educreations
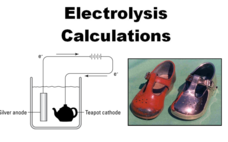
Electrolysis Calculations
Want to plate your baby shoe in copper? The video lesson explains the process of electroplating as a chemical procedure. Learners understand that electrolysis is the means to accomplish this goal.