Hi, what do you want to do?
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Concentration
Learn how concentration, evaporation, and saturation affect the concentration of a solution.
CK-12 Foundation
Ck 12: Solution Concentration
[Free Registration/Login may be required to access all resource tools.] Students will calculate the concentration of solutions in units of molarity, and use molarity to calculate the dilutions of solutions.
Texas Instruments
Texas Instruments: Determine Concentration of a Solution: Beer's Law
Students can use a Colorimeter to determine the relationship between concentration and absorbance of nickel sulfate solution (Beer's law). They will then determine the concentration of a unknown sample using the standard curve.
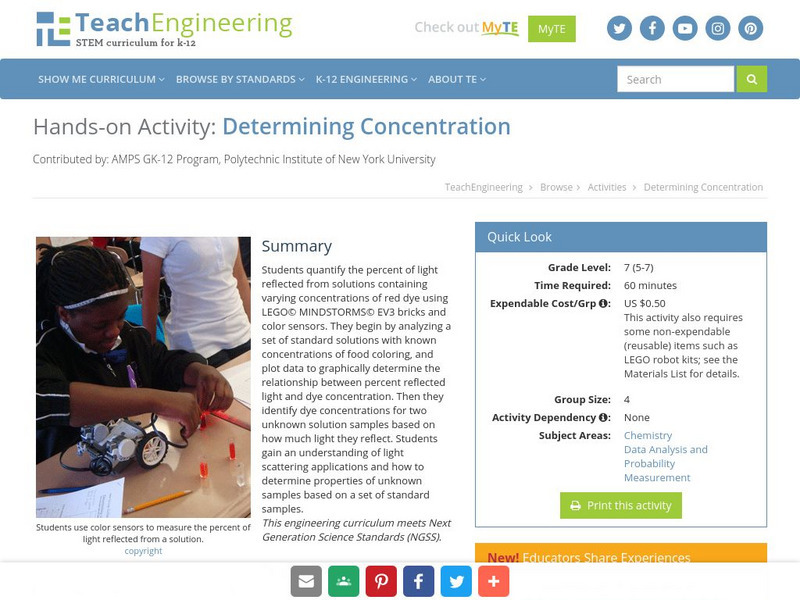
TeachEngineering
Teach Engineering: Determining Concentration
Students quantify the percent of light reflected from solutions containing varying concentrations of red dye using LEGO MINDSTORMS NXT bricks and light sensors. They begin by analyzing a set of standard solutions with known...
Sophia Learning
Sophia: Definition of Concentration
A narrated screencast which defines concentration, and illustrates some examples of different concentrations of solutions. [1:42]
Simon Fraser University
Chem1 Virtual Textbook: Solutions [Pdf]
With an overview of seven topics related to solutions, this .pdf file provides information on everything from osmosis and osmotic pressure to ions in aqueous solution. Other topics include solutions of volatile substances, methods of...
Chemistry Collective
Chem Collective: Creating a Stock Solution
In this activity, students use the virtual lab to create dilute solutions from a concentrated stock solution of acids or bases. They must first calculate the correct volumes of concentrated acid solution and water to mix together to...
Georgia Department of Education
Ga Virtual Learning: Chemistry: Solutions
In this module, students study solutions; how they are formed, how to calculate concentration of solutions, and what the colligative properties of solutions are.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: P H Scale Basics
In this simulation, students learn how changing the concentration of a solution affects the pH level.
TeachEngineering
Teach Engineering: Concentrate This! Sugar or Salt
Students investigate the property dependence between concentrations and boiling point. First, they investigate the boiling point of various liquid solutions. Then they analyze data collected from the entire class to generate two boiling...
BioEd Online
Bio Ed Online: What Is One Part Per Million Solution?
Substances dissolved in water can be present in very tiny amounts that are not visible to the eye. in this lesson students make a solution of food coloring with a concentration of one part per million.
Chemistry Collective
Chem Collective: Unknown Silver Chloride
Determine the concentration of silver ion in a Silver Nitrate solution using gravimetric analysis.
Khan Academy
Khan Academy: Spectroscopy: Interaction of Light and Matter
Tutorial provides a discussion of UV-Vis spectroscopy, infrared (IR) spectroscopy, and the Beer-Lambert law.
Chemistry Collective
Chem Collective: Cola and Sucrose Concentration Problem
In this activity, students use the virtual lab to prepare a sucrose solution for a soda recipe. They next calculate the concentration of their solution in terms of molarity, percent mass and density. Finally, they compare the density of...
Chemistry Collective
Chem Collective: Gravimetric Determination of Arsenic
Set in the context of ground water contamination in Bangladesh, this stoichiometry and analytical chemistry activity examines the issues around identifying wells contaminated with arsenic. In this activity, students perform a gravimetric...
Chem Tutor
Chem Tutor: Concentration
An overall summary of the major units of the concentration of a solution, including normality.
University of Colorado
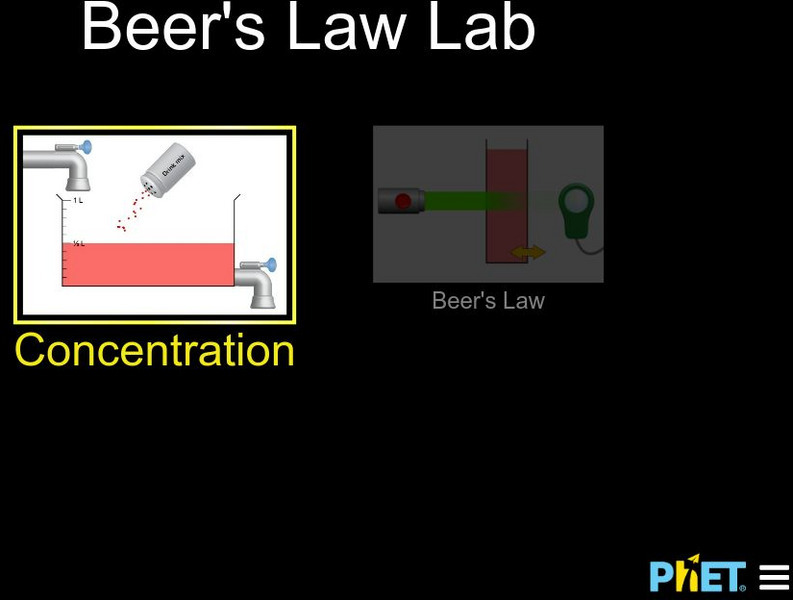
University of Colorado: Ph Et Interactive Simulations: Beer's Law Lab
"The thicker the glass, the darker the brew, the less the light that passes through." Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer!
Chemistry Collective
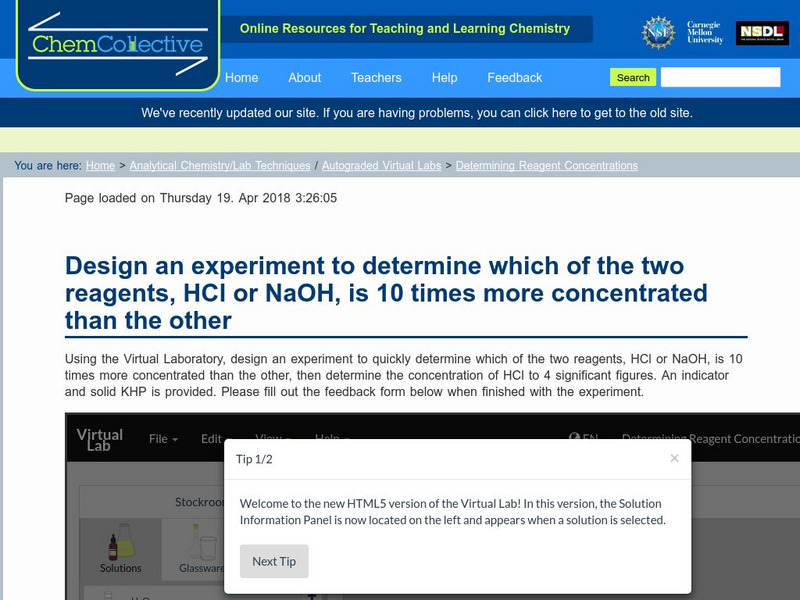
Chem Collective: Determining Reagent Concentrations
Using the Virtual Laboratory, design an experiment to quickly determine which of the two reagents, HCl or NaOH, is 10 times more concentrated than the other.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: P H Scale
In this advanced simulation, students experiment with changing the ion ratios in solutions and thus the pH. The experiment can be viewed on a macroscopic or microscopic level, or customized.
Georgia Department of Education
Ga Virtual Learning: Analysis of Organic Materials Analysis of Organic Materialsv
This comprehensive interactive tutorial continues to explore the forensic science field. Learn how organic materials of evidence are analyzed and what the various types of chromatography are, especially why they are so important to this...
Royal Society of Chemistry
Royal Society of Chemistry: Gridlocks: Level 3
A collection of grid puzzles that cover a wide variety of topics in advanced high school chemistry. These are excellent for topic review and reinforcement. The puzzles can be played online and also downloaded as worksheets. Answers are...
Chemistry Collective
Chem Collective: Determine the Concentration of the Unknown Strong Acid
Perform a titration using an indicator to determine the concentration of an HCl solution.
Chemistry Collective
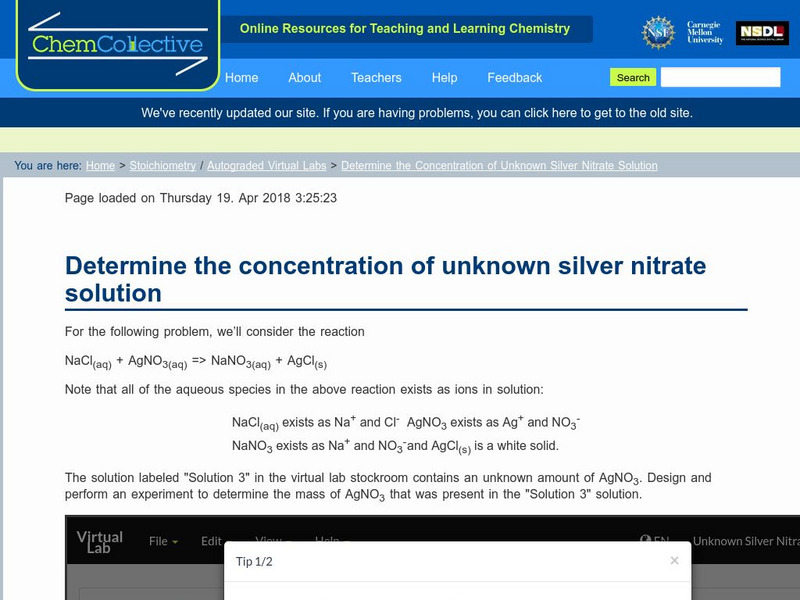
Chem Collective: Determine the Concentration of Unknown Silver Nitrate Solution
In this limiting reagents problem, students are asked to determine the amount of silver nitrate dissolved in a solution by performing a reaction with solid NaCl. In this randomized activity, each student is given a different unknown...
Science Struck
Science Struck: The Calibration Curve
Explains what Beer's Law and a spectrophotometer have to do with calibration curves. Describes the steps in making a calibration curve, by first making a set of standard solutions, then using a spectrophotometer to find the absorbance of...









![Chem1 Virtual Textbook: Solutions [Pdf] eBook Chem1 Virtual Textbook: Solutions [Pdf] eBook](https://static.lp.lexp.cloud/images/attachment_defaults/resource/large/FPO-knovation.png)