McMaster University
Mc Master University: Chemistry: Ionic Bonds
This site gives a brief description of bonding in ionic compounds.
American Chemical Society
Middle School Chemistry: Temperature Changes in Dissolving
See how temperature changes affect dissolving and the molecular bonds in a water molecule.
Other
Organic Chemistry: Lewis/kekule Structures
This slide presentation contains a few slides that will be useful for students learning to write Lewis structures. A discussion of multiple bonds is included, and clear examples are shown.
American Chemical Society
Middle School Chemistry: Temperature and Rate of a Chemical Reaction
Watch molecules collide with enough energy that bonds break and form new bonds in a chemical reaction.
American Chemical Society
Middle School Chemistry: Why Does Water Dissolve Sugar?
Explore this animation to learn why water dissolves sugar.
McMaster University
Mc Master University: Molecular Structure
This PowerPoint presentation features 33 slides that explain chemical bonding and molecular shape.
Other
Beautiful Chemistry: Beautiful Structures: Metal Organic Frameworks
Interact with these virtual structures of metal-organic frameworks. These special kinds of crystals are made of organic molecules and metal-containing units, which are linked together by chemical bonds.
American Chemical Society
Middle School Chemistry: Why Does Water Dissolve Salt?
Explore this animation to learn why water dissolves salt.
Other
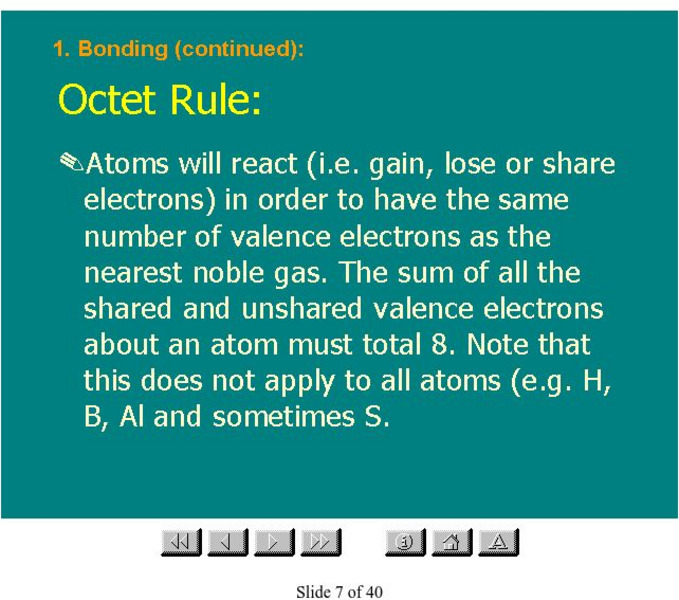
Organic Chemistry: Octet Rule
This slide presentation contains a description of the octet rule, as well as many related topics.
Curated OER
Simon Fraser University: All About Chemical Bonds
Irving Langmuir (1881-1967), chemist who won the Nobel Prize in 1932 for work on the chemistry of surfaces and monomolecular layers, and worked on the theory of electron pair bonding following N.G. Lewis.