Hi, what do you want to do?
Simon Fraser University
Chem1 Virtual Textbook: Chemical Energy
With an overview of topics related to chemical energetics, this site provides a foundation to a study of thermodynamics and molecules as energy carriers and converters. Topics covered include how molecules take up thermal energy,...
Other
Chemical Reax/exothermic Reactions
A wonderfully complete primer on exothermic reactions in the context of chemical spills. Covers Reactions with Air or Water, Combustible Organics, Polymerization Reax, Decomposition, Corrosion, and much much more.
Georgia Department of Education
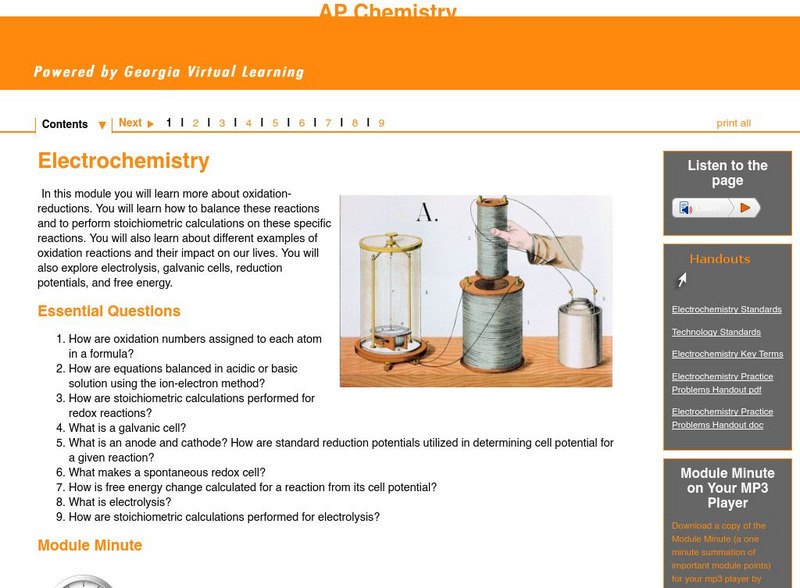
Ga Virtual Learning: Ap Chemistry: Electrochemistry
In this module students learn more about oxidation-reductions and how to balance these reactions and perform stoichiometric calculations on these specific reactions. Students also explore electrolysis, galvanic cells, reduction...
Other
The Chemistry Laboratory: A Lesson in Safety
A segment on safety in Chemistry that was written by Ira Remsen while he was at John Hopkins University.
Simon Fraser University
Chem1 Virtual Textbook: Reversible Reaction
Acting as an overview from the General Chemistry Virtual Textbook, this site seeks to answer the question, what is a reversible reaction? Acting as part of a larger informative site on equilibrium, various topics are addressed including...
National Association of Geoscience Teachers
Nagt: The Chemistry of Sand: Not All Beaches Are Created Equal
A laboratory activity where students investigate the chemistry of sand based on whether and how much it reacts with hydrogen chloride. They then make predictions and observe what happens when they do the same with various shell pieces.
American Chemical Society
Middle School Chemistry: What Is a Chemical Reaction?
Use chemical formulas to visualize how chemical equations are balanced.
Khan Academy
Khan Academy: Single Replacement Reactions
Definition of single replacement (or single displacement) reactions. Predicting and determining the products using the reactivity series.
CK-12 Foundation
Ck 12: Hess's Law and Standard Enthalpy of Formation
[Free Registration/Login may be required to access all resource tools.] Students will use Hess's law of heat summation to add chemical reactions together to produce a desired final equation, and then calculate the enthalpy change for...
CK-12 Foundation
Ck 12: Changes in Matter
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students identify the chemical properties of a substance and describe chemical changes and differentiate them from physical changes....
Science Bob Pflugfelder
Science Bob: Create Bubbles & Heat With Simple Chemistry
Use common supplies to try a simple experiment involving chemical reactions.
Frostburg State University
General Chemistry Online: Reactions in Solution
Resource provides a set of 18 slides that discuss chemical reactions in solution. Slides 15-18 discuss metathesis (double replacement) reactions, including formation of insoluble precipitate.
CK-12 Foundation
Ck 12: Organic Reactions
[Free Registration/Login may be required to access all resource tools.] Students distinguish between substitution reactions and addition reactions, and relate the concepts of oxidation and reduction to organic reactions.
Carnegie Mellon University
Chem Collective: Meals Ready to Eat
While camping on the Appalachian Trail, a storm dampens all the fire wood and you must design a chemical reaction to heat your meal. In this activity, students design an experiment in the virtual lab to determine the heat of reaction for...
CK-12 Foundation
Ck 12: Physical Science: Exothermic Reactions
[Free Registration/Login may be required to access all resource tools.] Explains the definition of an exothermic reaction, the role of energy, and examples of exothermic reactions.
ClassFlow
Class Flow: Chemical Reaction Classification
[Free Registration/Login Required] This is a basic flipchart for classification of simple chemical reactions. It includes sounds for feedback and use of Activotes for student self-assessment.
ClassFlow
Class Flow: Chemical Reactions
[Free Registration/Login Required] This flipchart discusses types of chemical reactions and their processes. There is a short Activote quiz included.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Oxidation Reduction Reactions: Audio Book
Listen to this audio book on Chapter 6, Oxidation-Reduction Reactions. View chapter maps, binary ionic compounds, memory aids, and examples of different chemical reactions.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Oxidation Reduction Reactions [Pdf]
Read about how oxidation-reductions affect our everyday lives. View how atoms, molecules, and ions are reduced during the oxidation-reduction process. Learn how some of the normal chemical reactions in the body can change DNA. Use the...
Dartmouth College
Dartmouth College: Chem Lab: Coordination Chemistry 1: Synthesis
In this four-week experiment, you will synthesize a compound and purify it in week 1. In week 2, you will assay it for purity by colorimetric analysis and by gravimetric analysis. In week 3, you will analyze your compound by titration of...
Concord Consortium
Concord Consortium: Stem Resources: Baggie Chemistry
Observe chemical and physical changes with this lab using everyday household items. Lab includes procedure and online data collection tool where answers can be saved and graded by teacher.
Alabama Learning Exchange
Alex: Chemistry Is Colorful!!!
In this lesson, learners are introduced to the 5 major types of chemical reactions. Each reaction type will be analyzed and specific examples will be noted. Students will then perform a lab investigation which includes each of the...
American Chemical Society
Middle School Chemistry: Forming a Precipitate
This diagram illustrates a chemical reaction forming a precipitate.
Estrella Mountain Community College
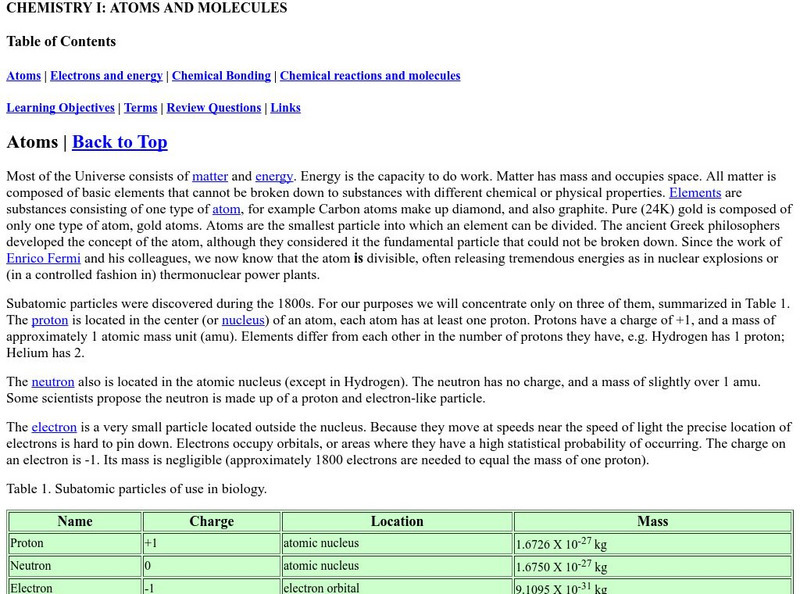
Online Biology Book: Chemistry I: Atoms and Molecules
In this online biology textbook, learn about atoms and molecules as they relate to life. Find out about topics such as electrons and energy, chemical bonding, and chemical reactions.