Hi, what do you want to do?
CK-12 Foundation
Ck 12: Fifth Grade Science: Physical Science: Chemical Properties of Matter
A module that provides the definition of chemical property and examples of the chemical properties of matter using explanations, pictures, and review questions.
SMART Technologies
Smart: Physical and Chemical Properties
Students will learn the difference between physical and chemical properties of matter.
Utah State Office of Education
Utah Science: Changing Matter
Matter changes both physically and chemically on a fairly regular basis. Through these activities students will challenge their understanding of the changes that may take place with different types of matter.
American Chemical Society
Middle School Chemistry: Lesson Plans: Using Chemical Change to Identify Unknown
Students observe reactions of liquids with different known powders in this lesson. Unknown powders are then identified using characteristic chemical changes.Site includes a procedure, teacher instructions, and video instruction.
Frostburg State University
General Chemistry Online: Ten Signs of Chemical Change
Resource provides the ten signs that tell when a chemical change has occured. Each sign has a detailed explanation.
American Chemical Society
American Chemical Society: Inquiry in Action: Teach Science Well
Online textbook reviews fundamentals of chemistry and physical science via slideshow presentations, notes, and videos. Materials for classroom activities engage students in inquiry-based, hands-on investigations covering molecular...
American Chemical Society
American Chemical Society: Inquiry in Action: Teach Science Well
Online textbook reviews fundamentals of chemistry and physical science via slideshow presentations, notes, and videos. Materials for classroom activities engage students in inquiry-based, hands-on investigations covering molecular...
Science Education Resource Center at Carleton College
Serc: Investigate Chemical Changes: What Are Some Signs of Chemical Change?
This lab illustrates the condition of color change and the formation of gas (bubbles) as a result of a chemical change. Students will be able to name the characteristics of chemical changes.
TeachEngineering
Teach Engineering: All Fat Is Not Created Equally!
Students learn that fats found in the foods we eat are not all the same; they discover that physical properties of materials are related to their chemical structures. Provided with several samples of commonly used fats with different...
TeachEngineering
Teach Engineering: Mixtures and Solutions
This unit covers introductory concepts of mixtures and solutions. Students think about how mixtures and solutions, and atoms and molecules can influence new technologies developed by engineers. The first lesson explores the fundamentals...
Frostburg State University
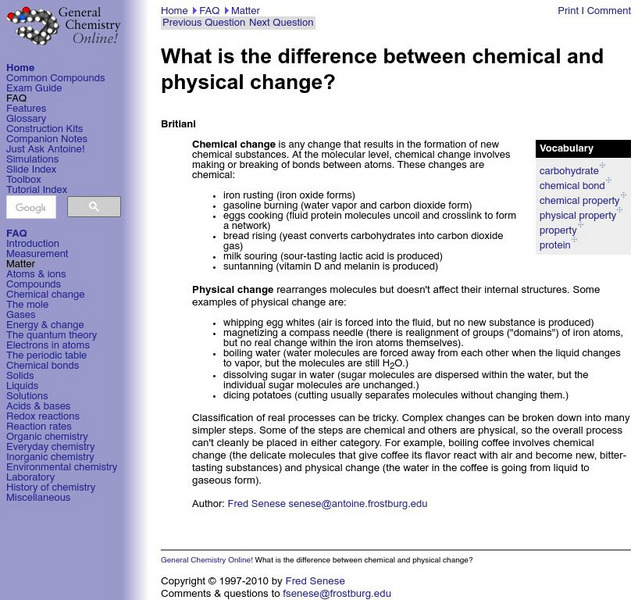
General Chemistry Online: Physical and Chemical Changes
Resource provides information about the difference between physical and chemical changes. Includes a definition and examples of each.
CK-12 Foundation
Ck 12: Chemistry: Physical Change
[Free Registration/Login may be required to access all resource tools.] Covers reversible and irreversible physical changes.
Sophia Learning
Sophia: Physical and Chemical Changes
This lesson will describe physical and chemical properties and changes of matter.
CK-12 Foundation
Ck 12: Chemistry: Physical Change
[Free Registration/Login may be required to access all resource tools.] Definition of physical change and examples, and how physical changes can be reversed.
Science Education Resource Center at Carleton College
Serc: Observing,describing, Measuring Changing Physical Prop: Making Ice Cream
Students will create an endothermic chemical reaction by making ice cream. They will observe, describe, and measure the ingredients. After making ice cream, students will measure the temperature of the ice cream and the ice/salt mixture....
Other
Elmhurst College: Virtual Chembook: What Are Physical Properties and Changes?
Brief descriptions of physical properties, physical changes, and the process of sublimation. The three states of matter, melting point, and boiling point are described. There is one link on the page, which leads to an explanation of...
Savvas Learning
Chemistry: Central Science Live: Properties of Solutions
This site provides an excellent overview of the solution process. Content includes a focus on how a solution is formed, energy changes and solution formation, spontaneity and disorder, and solution formation and chemical reactions.
CK-12 Foundation
Ck 12: Changes in Matter
[Free Registration/Login may be required to access all resource tools.] Students will describe methods for separating mixtures, such as chromatography, distillation, fractional distillation, evaporation, and filtration. They will also...
Other
Study Stack: Changes in Matter
An online flashcard deck that reviews properties of matter. Test your knowledge of matter, chemical changes, and physical changes. Site allows you to put flashcards into two sections, those you know and those you don't. After separating...
Science Education Resource Center at Carleton College
Serc: Plastic Polymers: Investigating Their Flexibility
Students will use their prior knowledge about changes of matter to develop a hypothesis to test the physical properties of materials such as plastic (polymers) and how its chemical properties allow it to have unique physical properties.
Alabama Learning Exchange
Alex: What's the Matter: Concentration Game
What's the Matter? is a guided inquiry lesson on classification of matter, physical and chemical properties, and physical and chemical changes. There are two parts to this lesson: (a) concentration game for definitions and (b)...
Read Works
Read Works: Everyday Compound or Poison?
[Free Registration/Login Required] An informational text about how chemicals can be combined to form safe or unsafe compounds. A question sheet is available to help students build skills in reading comprehension.
Concord Consortium
Concord Consortium: Stem Resources: Baggie Chemistry
Observe chemical and physical changes with this lab using everyday household items. Lab includes procedure and online data collection tool where answers can be saved and graded by teacher.
Science Education Resource Center at Carleton College
Serc: Chemical Changes and Healthy Bodies All From Snot!
In this demonstration, students observe the properties of "snot"; determine any changes within the "snot"; record and discuss their findings. This demonstration is done within a unit on the respiratory system. It leads to lively...