Hi, what do you want to do?
Curated OER
Atomic Electron Configuration and Chemical Periodicity
For this electron configuration and periodicity worksheet, students answer fifteen questions about periodic trends and they analyze orbital diagrams indicating location of electrons.
National Institute of Open Schooling
Chemical Bonding
Name is Bond, covalent bond. Through readings and answering questions, classes explore the different types of chemical bonds, their characteristics, valence shell electron pair repulsion theory, and atomic orbitals.
Curated OER
2002 U.S. National Chemistry Olympiad National Exam - Part I
As to be expected from the American Chemical Society Olympiad Examinations Task Force, this 60-question test tops the charts in terms of excellence. It consists entirely of multiple choice questions designed to assess a year's worth of...
Curated OER
Electron Arrangement Worksheet
In this electron activity students complete an orbital diagram and electron configuration using a set of chemicals on the periodic table.
Curated OER
Chemistry Jeopardy
In preparation for an introduction to chemistry exam, play Chemistry Jeopardy with your class. The categories include molar relationships, phases of matter, atomic structure, chemical nomenclature, and scientific investigation. If the...
Curated OER
Atom Basics Test
Simple in format and standard in content, this resource is an assessment of your beginning chemists' grasp of the atom. Using a periodic table of elements, they fill in a chart of missing chemical formulas, atomic masses, and numbers of...
National Institute of Open Schooling
Atomic Structure
Learners explain historical findings such as Rutherford and Bohr's contributions, explain wave particle duality, and formulate Heinsenberg's uncertainty principle. They also draw s, p, and d orbitals, explain more historical findings,...
Virginia Department of Education
Elements and Electron Configuration
It's electronic! Pupils uncover elements and their electron configurations as they explore mass, groupings, correct charges, and sliding theory. Young scientists learn creative ways to remember various elements and correctly...
Virginia Department of Education
Atomic Structure: Periodic Table
The fifth lesson of seven in the series outlines an in-depth analysis of the periodic table. After direct instruction, pupils take turns practicing in the group before beginning independent study. The assessments include a...
Curated OER
Free Radical Reactions
In this chemistry worksheet, young scholars look at the diagrams for the chemistry reaction. Then they write down the correct chemical reaction.
Virginia Department of Education
Atomic Structure: Elements
It's all relevant, really. Individuals use the scientific method to learn more about elements, atoms, and their placement on the periodic table. They conduct experiments using materials common in nature to explore how elements affect our...
Curated OER
The Sun Affects Earth
Third graders read, write, and listen to information about the sun and its effects on the Earth.as it relates to its axis, orbit, rotate, and revolution. In this solar system lesson, 3rd graders examine how the sun...
Curated OER
Order, Order All Electrons
Students read the periodic table and apply their knowledge of the construction of atoms. They demonstrate reading the electron configuration of an element on the periodic file.
Simon Fraser University
Chem1 Virtual Textbook: Molecular Orbitals
Acting as an overview from the General Chemistry Virtual Textbook, this site explores the topics related to molecular orbitals including bonding and antibonding MOs, molecular orbital diagrams, sigma and pi orbitals, and more.
Michael Blaber, PhD
Fsu: Basic Concepts of Chemical Bonding: Exceptions to the Octet Rule
Lists the exceptions to the octet rule and provides a discussion and diagrams explaining each one. Includes clear diagrams illustrating this concept.
Other
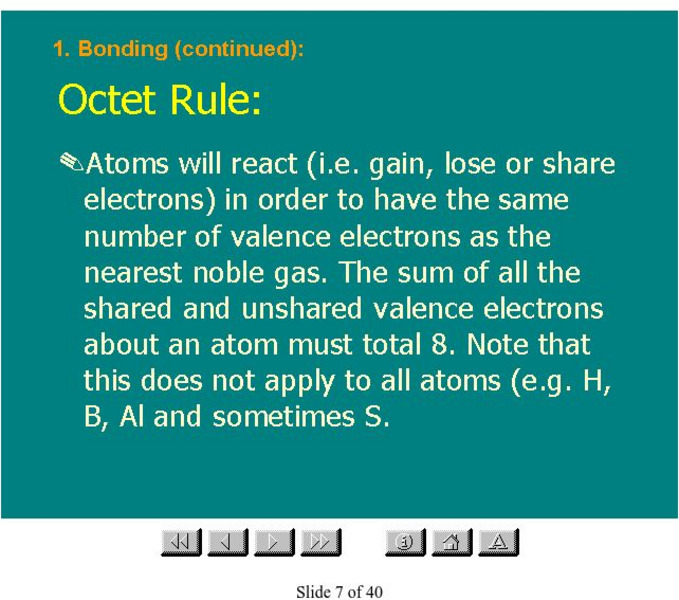
Organic Chemistry: Octet Rule
This slide presentation contains a description of the octet rule, as well as many related topics.