Hi, what do you want to do?
Sophia Learning
Sophia: Hydrogen Bonding Definition: Lesson 1
This lesson will define hydrogen bonding. It is 1 of 2 in the series titled "Hydrogen Bonding Definition."
Ducksters
Ducksters: Chemistry for Kids: Chemical Bonding
Study chemical bonding in chemistry including atoms, valence electrons, ionic and covalent bonding, and how molecules are formed on this site!
Khan Academy
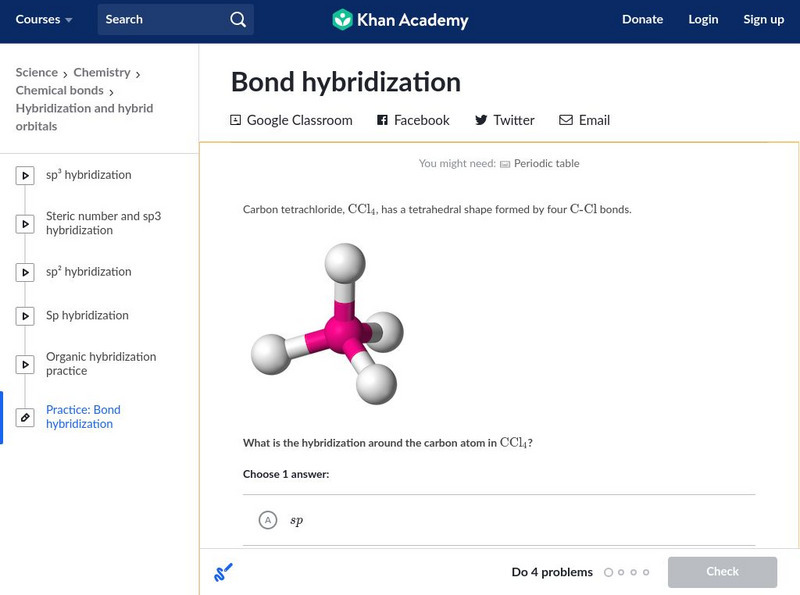
Khan Academy: Bond Hybridization
Practice determining the hybridization for atoms in covalent compounds. Use the interactive scratch pad to work out the problems.
Khan Academy
Khan Academy: Single and Multiple Covalent Bonds
Explains what covalent bonds are and the difference between them and ionic bonds. Discusses the properties of polar and non-polar covalent bonds, and single and multiple covalent bonds, and gives analogies and examples of how covalent...
Sophia Learning
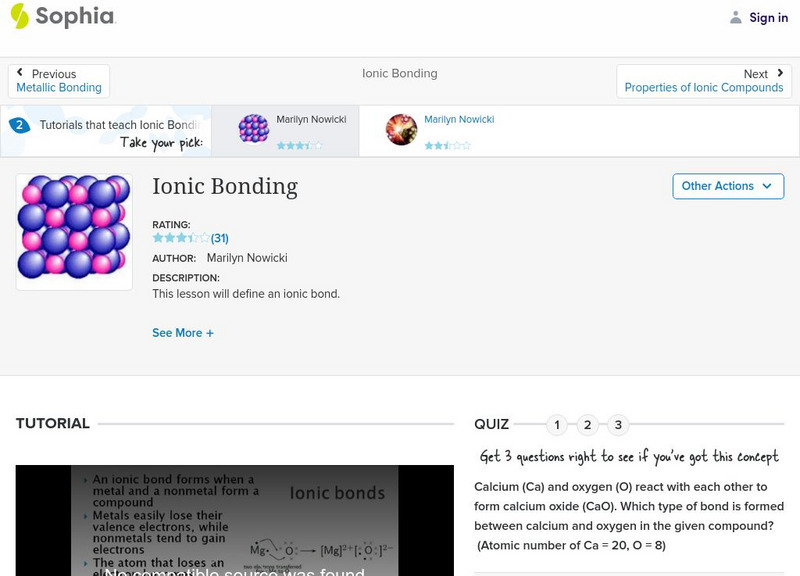
Sophia: Ionic Bonding: Lesson 2
This lesson will define an ionic bond. It is 2 of 3 in the series titled "Ionic Bonding."
Khan Academy
Khan Academy: The Chemical Structure Consequences of Beta Lactams
The Chemical Structure Consequences of Beta-lactams.
American Chemical Society
Middle School Chemistry: Chapter 4: The Periodic Table and Bonding
Six middle school chemistry lessons about the periodic table and bonding complete with handouts and animations.
National Institutes of Health
Ncbi: The Molecular Biology of the Cell: The Chemical Components of a Cell
Advanced chapter of the book "The Molecular Biology of the Cell" describes and provides illustrations of our most current understanding of the chemical makeup of cells and their components. Explains in detail how electron activity keeps...
Utah Education Network
Uen: Classroom Bonding
Students will be assigned an ion "name tag" and move around the classroom finding suitable other "atoms" to bond with.
Science Struck
Science Struck: Characteristics of Ionic Bonding
Explains what chemical bonds are and how they form, the three most common types, and the characteristics of ionic bonds. Gives an example of ionic bonding.
Michael Blaber, PhD
Fsu: Basic Concepts of Chemical Bonding: Exceptions to the Octet Rule
Lists the exceptions to the octet rule and provides a discussion and diagrams explaining each one. Includes clear diagrams illustrating this concept.
Simon Fraser University
Chem1 Virtual Textbook: Hybrid Types and Multiple Bonds
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. Acting as a second part to a discussion on hybrid types of bonding, this site specifically...
American Chemical Society
Middle School Chemistry: Energy Changes in Chemical Reactions
Students will conduct two chemical reactions: endothermic and exothermic. They will see an animation that shows that it takes energy to break bonds and that energy is released when new bonds are formed, and use that animation to explain...
American Chemical Society
American Chemical Society: Hompage
ChemCenter, available from the American Chemical Society, provides chemistry news, reference sources and other public services.
Concord Consortium
The Molecular Workbench Database: Chemical Reactions and Stoichiometry
This simulation helps students investigate the basics of chemical equations. Students will review stoichiometry and what effects their reaction rates.
Concord Consortium
The Molecular Workbench Database: Energy Conservation in Chemical Reactions
Follow the energy conversion from potential to kinetic in this interactive activity that explores total energy in chemical reactions.
Science Education Resource Center at Carleton College
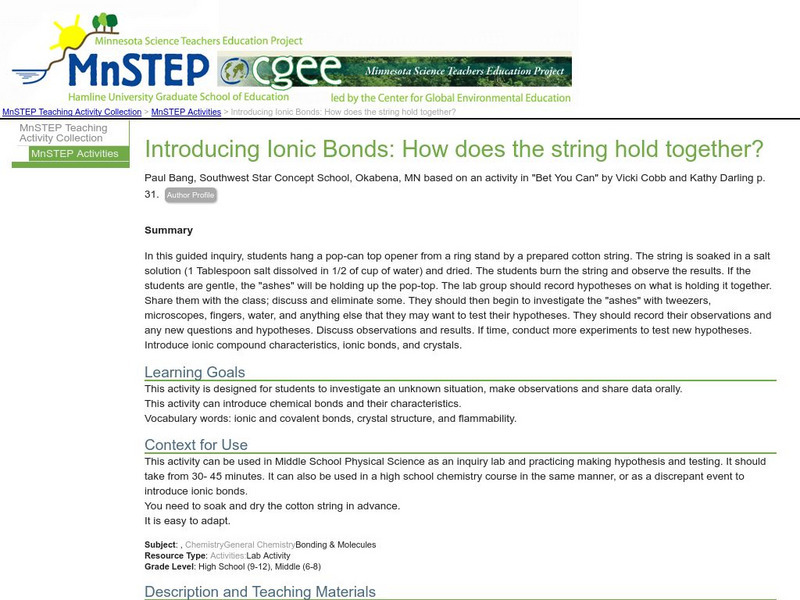
Serc: Introducing Ionic Bonds: How Does the String Hold Together?
This inquiry activity introduces chemical and ionic bonds and allows students to investigate an unknown situation, make hypotheses, and share data.
American Chemical Society
Middle School Chemistry: Temperature and Rate of a Chemical Reaction
Watch molecules collide with enough energy that bonds break and form new bonds in a chemical reaction.
Concord Consortium
The Concord Consortium: Molecular Workbench: Seeing Chemical Equilibrium
Observe a visual representation of the equilibrium of products and reactants in chemical reactions. Record data while the reactions are taking place and print out a report afterwards.
CPALMS
Florida State University Cpalms: Florida Students: Physical and Chemical Changes
Explore the differences between chemical and physical changes, including some examples at the atomic level.
Other
Chemical Bonds: Writing Lewis Structures
This slide show explains the rules for writing Lewis structures and then demonstrates an example. It then provides a practice example for the student to try.
Nobel Media AB
The Nobel Prize: The Nobel Prize in Chemistry 1954
Read about Linus Carl Pauling (1901-1994), the recipient of two Nobel Prizes, and his research into the nature of the chemical bond which earned him recognition in the world of chemistry.
McMaster University
Mc Master University: Chemistry: Ionic Bonds
This site gives a brief description of bonding in ionic compounds.
Other
Chemical Bonds: Electronegativity
A slideshow with several slides dedicated to electronegativity, the trend which is illustrated in the slide at this link. Others slides explain how the electronegativity can be used to determine bond character.
Other popular searches
- Types of Chemical Bonds
- Covalent Chemical Bonds
- Chemical Bonds Lab
- Chemical Bonds Biology
- Chemical Bonds and Reactions
- Review Chemical Bonds
- Chemistry Chemical Bonds
- Chemical Bonds Review Games
- Science, Chemical Bonds
- Chemical Bonds in Milk
- Science Chemical Bonds
- Chemical Bonds in Liquids