National Institute of Open Schooling
Chemical Bonding
Name is Bond, covalent bond. Through readings and answering questions, classes explore the different types of chemical bonds, their characteristics, valence shell electron pair repulsion theory, and atomic orbitals.
National Institute of Open Schooling
Chemical Thermodynamics
All chemical reactions require energy. To explore thermodynamics, classes read and discuss its laws, exothermic and endothermic reactions, enthalpy in many forms, calculate enthalpy problems, and use Hess' Law to calculate enthalpy of a...
National Institute of Open Schooling
Coordination Compounds
Cyanide, a coordination compound, is used in the extraction of gold and silver. Part 24 in the series of 36 delves into the world of coordination compounds. Classes learn, through readings, discussions, and answering questions, how to...
National Institute of Open Schooling
Aldehydes, Ketones and Carboxylic Acids
Although their name makes them sound dangerous or toxic, carboxylic acids are found throughout nature in things such as citric acid, vinegar, and even in your DNA. Through detailed readings, discussions, and answering questions...
It's About Time
Organic Substances
Host an exciting lab in which learners burn fruit rinds to better understand hydrocarbons. A reading passage and analysis questions wrap up the lesson.
National Institute of Open Schooling
p-Block Elements and Their Compounds – II
Ozone, made of three bonded oxygen atoms, is found 15-30 km above Earth, has a strong smell, is blue, and blocks sunlight from hitting the surface of Earth. The 22nd lesson in a series of 36 specifically focuses on the important elements...
National Institute of Open Schooling
Alcohols, Phenols and Ethers
Classes continue their study of organic compounds in a detailed lesson covering alcohols, phenols, and ethers. Naming these compounds, classifying them, and describing their preparation and use are some of the topics covered. Through...
American Chemical Society
Development of Baking Powder
Did you know baking powder can be used to treat acne, whiten teeth, and make sugar cookies? The lesson on the development of baking powder is ready-to-go with no preparation required. Through readings, pupils answer questions, complete...
National Institute of Open Schooling
Nomenclature and General Principles
Carbon, the base for all organic compounds, exists in nature in its purest form as graphite or diamonds. The 25th lesson in a series of 36 teaches pupils the nomenclature of organic compounds. Learners read about how to use the IUPAC...
National Institute of Open Schooling
General Characteristics of the p-Block Elements
The 20th installment in a series of 36 focuses on the characteristics of the p-block elements. Learners discuss, read about, and answer questions pertaining to the occurrence of these elements in nature, their electron configurations,...
National Institute of Open Schooling
Atomic Structure
Learners explain historical findings such as Rutherford and Bohr's contributions, explain wave particle duality, and formulate Heinsenberg's uncertainty principle. They also draw s, p, and d orbitals, explain more historical findings,...
National Institute of Open Schooling
Periodic Table and Atomic Properties
An in-depth lesson, the fourth activity in a series of 36, begins with teaching how the periodic table's arrangement came to its current design. Using this knowledge, pupils then move on to analyze the arrangement of elements to their...
CK-12 Foundation
Ck 12: Earth Science: Chemical Bonding Study Guide
Understand chemical boding using this review guide.
Simon Fraser University
Chem1 Virtual Textbook: Molecules and the Properties of Bonded Atoms
College-level site goes into great detail about chemical bonding properties, as far as energies, structure, angles, and length. Infrared absorption, the greenhouse effect, and global warming are addressed to exemplify bond length...
Frostburg State University
General Chemistry Online: Chemical Bonds Faq
Find answers to common questions about chemical bonds. Recognize molecular orbital theory and know how to draw a Lewis structure.
University of Florida
University of Florida: General Chemistry I: Chemical Bonding
Notes on covalent bonding, bond length, bond energies, Lewis dot structures, and percent ionic character. Colorful graphics.
Oklahoma State University
Osu: Chemical Bonding Content in a Nutshell
An overview of chemical bonding and how valence electrons play a major role in these processes.
Simon Fraser University
Chem1 Virtual Textbook: Bonding in Metals and Semiconductors
Acting as an overview from the General Chemistry Virtual Textbook, this site explores chemical bonding in relation to metals and semiconductors. Topics covered include molecular orbitals in metals, band structure in metals, and more.
Michael Blaber, PhD
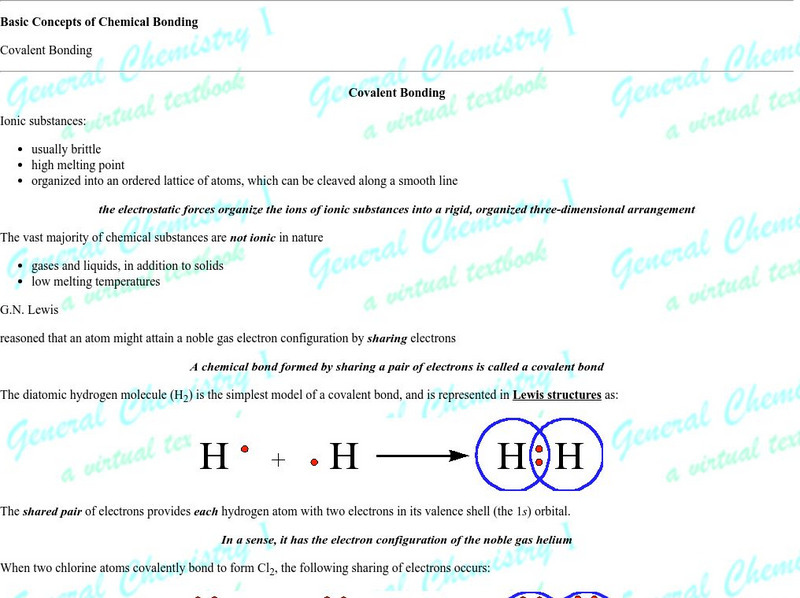
Florida State University: Basic Concepts of Covalent Bonding: Covalent Bonding
Good introduction and graphics make this a solid page for understanding the orbital role in bonding and molecular geometry. The author is a professor at Florida State University.
Michael Blaber, PhD
Fsu: Basic Concepts of Chemical Bonding: Polarity and Electronegativity
Florida State University provides an explanation of electronegativity of elements and its affect on bond type.
Science4Fun
Science4 Fun: Chemical Bonding
What is a chemical bond? Learn about the structure of an atom, why bonds are formed, and the different types of chemical bonds.
National Institutes of Health
Ncbi: The Molecular Biology of the Cell: The Chemical Components of a Cell
Advanced chapter of the book "The Molecular Biology of the Cell" describes and provides illustrations of our most current understanding of the chemical makeup of cells and their components. Explains in detail how electron activity keeps...
Science Struck
Science Struck: Characteristics of Ionic Bonding
Explains what chemical bonds are and how they form, the three most common types, and the characteristics of ionic bonds. Gives an example of ionic bonding.
Michael Blaber, PhD
Fsu: Basic Concepts of Chemical Bonding: Exceptions to the Octet Rule
Lists the exceptions to the octet rule and provides a discussion and diagrams explaining each one. Includes clear diagrams illustrating this concept.
Other popular searches
- Chemical Bonding Lesson Plan
- Chemical Bonding Crossword
- Chemical Bonding Activity
- Edible Lab Chemical Bonding
- Chemistry Chemical Bonding
- Chemical Bonding Ionic
- Chemical Bonding Inquiry
- Worksheets Chemical Bonding
- Chemical Bonding Case Study
- Chemistry Chemical Bonding
- Matter and Chemical Bonding
- Science Chemical Bonding