Curated OER
Chem 1: The Shell Model of the Atom
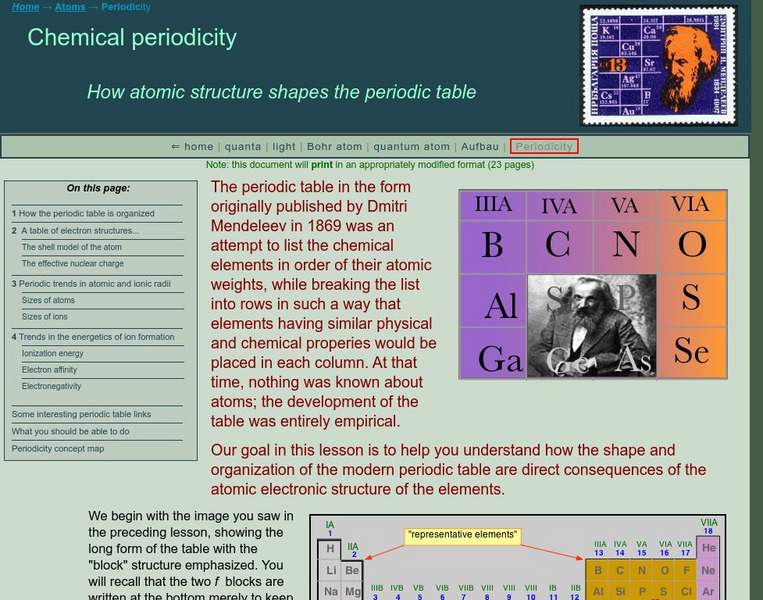
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
Curated OER
Shell Model of the Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
Curated OER
Core Charge of an Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
Other
Atoms in Motion: All Matter Is Made of Atoms
Atoms are very, very small. Atoms are so small that it is often said that there are as many atoms in a single grain of sand as there are grains of sand on all of the world's beaches - certainly a difficult thing to prove, but you get the...
Simon Fraser University
Chem1 Virtual Textbook: Effective Nuclear Charge
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the core charge of the atom, otherwise termed the nuclear charge.
Simon Fraser University
Chem1 Virtual Textbook: Spectrum of a Guitar String
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses spectrum in relation to Bohr's model. Included in the topics covered are standing waves, boundary condition,...
Simon Fraser University
Chem1 Virtual Textbook: Electronegativity
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses shared chemical bonds between two elements resulting in electronegative and electropositive outcomes.
Other
Characteristics of Energy and Matter
A lengthy page from the Fundamentals of Physical Geography site. Energy is distinguished from matter, and the different forms of energy are identified and discussed. Four types of heat transfer (convection, advection, conduction,...
Curated OER
Concept Map Particles and Waves
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses wavelength and the uncertainty principle. Information is also provided on de Broglie wavelength and electron...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Cation Names and Formulas
Learn how to predict cation names, charges, and formulas. See how you can use the periodic table to help predict what metallic atoms will do.
Science4Fun
Science4 Fun: Elements
Fun and interesting information about elements. Learn about the periodic table, how they are distinguished, and the different families of elements.
Simon Fraser University
Chem1 Virtual Textbook: Wave, Particle, or What?
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses light, waves, and particles. Part of the discussion involves a definition and working information on the...
Ducksters
Ducksters: Chemistry for Kids: Elements: The Noble Gases
Study the noble gases on this site. Learn about where to find them on the periodic table, what elements are in this group, and other facts.
Ducksters
Ducksters: Chemistry for Kids: Elements: Lanthanides and Actinides
Kids learn about the lanthanides and actinides of the periodic table. Find out which elements are in this group. properties, similarities, and other facts on this site.
American Chemical Society
American Chemical Society: Hompage
ChemCenter, available from the American Chemical Society, provides chemistry news, reference sources and other public services.
Curated OER
Atomic Radii Definitions
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
Curated OER
Ionic Radii Measurement
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
Curated OER
Covalent Atomic Radii Periiodic Trends
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
Curated OER
Ionic Radii Periiodic Trends
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
Curated OER
Isoelectronic Series
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
Curated OER
Ionization Energies Periiodic Trends
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
Curated OER
Electron Affinities Periiodic Trends
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
CK-12 Foundation
Ck 12: Physical Science: Valence Electrons
[Free Registration/Login may be required to access all resource tools.] Valence electrons, their variation in the periodic table and relation to reactivity and electrical conductivity of elements.
BBC
Bbc: Gcse Bitesize: Atomic Structure
This lesson focuses on early ideas about the structure of an atom including John Dalton's plum pudding model and Ernest Rutherford's nuclear model. A link to a test is provided.