Other
University of Kansas: Quarked!: Matter Mechanic
Build elements and molecules using neutrons, protons, and electrons. Choices include helium, carbon, oxygen, aluminum, water, and salt.
Sophia Learning
Sophia: Subatomic Particles: Lesson 2
Describe the $ifference between the subatomic particles, including their masses, locations, and charges. This lesson is 2 of 7 in the series titled "Subatomic Particles."
Curated OER
Science Kids: Science Images: Basic Atom Structure
This diagram shows a basic atom structure with an atom core, proton, neutron and electron.
Lawrence Berkeley National Laboratory
Berkeley Lab: La Aventura De Las Particulas
Learn the fundamentals of particles and forces with this site. Explore the paths that explain matter in the universe.
Mocomi & Anibrain Digital Technologies
Mocomi: Molecules
When two or more atoms combine they give rise to a molecule. A molecule can be made up of many atoms of the same element. There are 112 elements known to man, so can you imagine the different permutations and combinations of molecules...
Thomas Jefferson National Accelerator Facility
Jefferson Lab: It's Elemental Element Math Game!
Learn how to read the periodic table of elements as you solve these Math questions about the number of protons, neutrons, electrons or nucleons in an atom of an element. You can choose how many questions to answer, and how complex they...
Annenberg Foundation
Annenberg Learner: Interactives: The Periodic Table
An interactive website where students learn about the basics of an atom, periodic tables organization, and the structure and properties of matter. Module includes an introduction and five lessons that are followed by a quiz and an...
Annenberg Foundation
Annenberg Learner: Interactive Periodic Table of the Elements
A fun way to learn about the periodic table! This interactive table allows students to investigate the basic information of an element as well as explore group and family characteristics.
Nobel Media AB
The Nobel Prize: The Nobel Prize in Physics 1935 Presentation Speech
The Nobel Physics Chairman made this speech when presenting the Prize to Chadwick. It clearly explains the importance and depth of Chadwick's work. Site by Nobel e-Museum.
American Chemical Society
Middle School Chemistry: Periodic Table
Students explore the periodic table and learn the basic information given for elements: the name, symbol, atomic number, and atomic mass for each element.
Utah Education Network
Uen: Atomic Model Construction
Students create models of atoms then compare the various aspects of the atoms including; relative size, charge, positions of subatomic particles, and identity of the atom based on proton, neutron, and electrons with the class.
Wikimedia
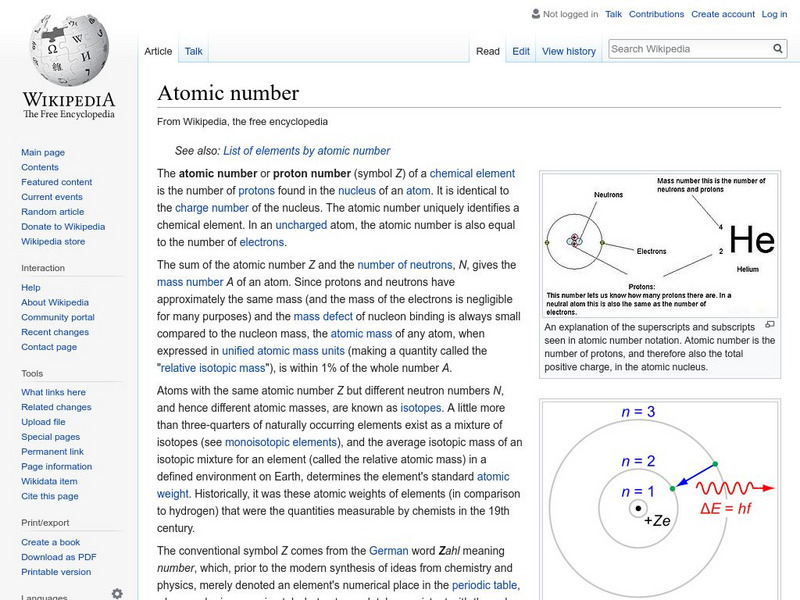
Wikipedia: Atomic Number
Wikipedia provides the definition of the term, "Atomic number," a term used in chemistry and physics to represent the number of protons in the nucleus of an atom.
Sophia Learning
Sophia: Isotopes: Lesson 9
This lesson will define an isotope and explain what happens if the number of neutrons in an atom changes. It is 9 of 9 in the series titled "Isotopes."
Sophia Learning
Sophia: Isotopes: Lesson 1
This lesson will define an isotope and explain what happens if the number of neutrons in an atom changes. It is 1 of 9 in the series titled "Isotopes."
Sophia Learning
Sophia: Subatomic Particles: Lesson 3
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 3 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: Lesson 4
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 4 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: Lesson 6
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 6 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: Lesson 7
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 7 of 7 in the series titled "Subatomic Particles."
Physics4kids
Physics 4 Kids: Where Traditional Physics Stops
We're about to move into the modern age of physics. In the early 1800's, scientists began examining the basis of matter, space, and time. Sometimes it gets very confusing, but the big idea is that Newton's physics describe about 90% of...
Sophia Learning
Sophia: Subatomic Particles: Lesson 5
Describe the difference between the subatomic particles, including their masses, locations, and charges. This lesson is 5 of 7 in the series titled "Subatomic Particles."
Physics Classroom
The Physics Classroom: Static Electricity Review
This review from the Glenbrook South High School provides a series of questions on various topics associated with static electricity (such as electrical insulation). Answers and explanations are hidden, yet easily accessed from within a...
Lawrence Berkeley National Laboratory
Berkeley Lab: Basic Nuclear Science Information
Site provides the ABC's of nuclear science including radioactivity and gamma decay to fission and comic rays.
Sophia Learning
Sophia: Atomic Mass: Lesson 4
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. Module includes a slideshow and a quiz.
Concord Consortium
Concord Consortium: How Does an Object Become Charged?
Activity 1 in this module: What is the effect of changing the composition of an atom? Since all atoms contain protons, neutrons, and electrons, what makes one element different from another is examined.