CK-12 Foundation
Ck 12: Isotopes and Atomic Mass
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will be asked to define atomic number, mass number and understand how isotopes differ from one another. They will be able...
CK-12 Foundation
Ck 12: Mass and the Mole
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will use molar mass to make conversions between mass and moles of a substance and they will also use the mole road map to...
CK-12 Foundation
Ck 12: Physical Science: Atomic Number
[Free Registration/Login may be required to access all resource tools.] Definitions of atomic number and mass number.
CK-12 Foundation
Ck 12: Physical Science: Atomic Number
[Free Registration/Login may be required to access all resource tools.] Definitions of atomic number and mass number.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Isotopes and Atomic Mass
Are all atoms of an element the same? How can you tell one isotope from another? Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element.
Texas Education Agency
Texas Gateway: Ap Physics I: Appendices: Atomic Masses
This is a chart of the elements including the atomic mass, atomic number, symbol, half-life, and more.
Science Education Resource Center at Carleton College
Serc: Investigating Isotopes: Using M&m's as a Model for Calculating Atomic Mass
This activity allows students to gain an understanding of the relationship between the mass and relative abundance of an isotope and its effect on the average atomic mass of the element. Students will be given a random sample of the...
Science Education Resource Center at Carleton College
Serc: The Mole in Chemistry: Determining the Number of Atoms in Everyday Items
Students explore the concept of the mole as a measure of quantity. They build spreadsheets that allow them to convert between moles, grams, and atomic mass units for given quantities of everyday items, elements, and simple chemical...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Relating Mass to Number of Particles: Audio Book
This slide and audio book shows how to relate mass to a number of particles by using many formulas, examples, and real life scenarios. View the weighted average calculations, Avogadro's number, the atomic mass of carbon, and molar mass.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Isotopes and Atomic Mass
Are all atoms of an element the same? How can you tell one isotope from another? Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element.
Science Education Resource Center at Carleton College
Serc: Investigating Isotopes and Average Atomic Mass Using Pennies
In this activity, students apply what they have learned about weighted averages, isotopes, and systems of equations to a new situation - coins in a sealed container. They learn some historical information regarding the composition of...
Environmental Chemistry
Environmental chemistry.com: Anatomy of an Atom
Explains the basics of atomic structure, from simple definitions to information about quantum theory. Accurate and helpful basics whether or not you need the more advanced information.
CK-12 Foundation
Ck 12: Chemistry: Calculating Atomic Mass
[Free Registration/Login may be required to access all resource tools.] Covers definition of atomic mass and calculations of atomic mass.
CK-12 Foundation
Ck 12: Chemistry Simulation: Average Atomic Mass
[Free Registration/Login Required] Students explore how percent abundances of different isotopes affect the average atomic mass for that given element. Students form connections between the average atomic mass and a person's body mass....
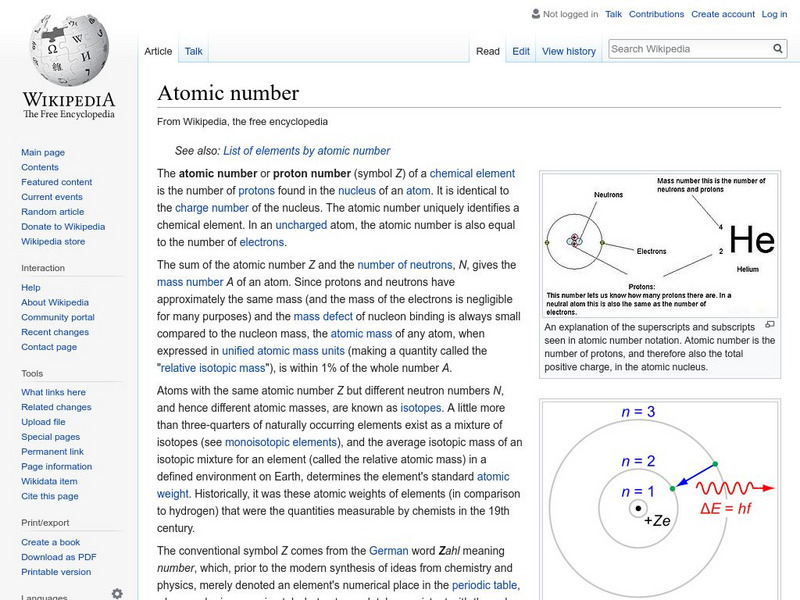
Wikimedia
Wikipedia: Atomic Number
Wikipedia provides the definition of the term, "Atomic number," a term used in chemistry and physics to represent the number of protons in the nucleus of an atom.
Sophia Learning
Sophia: Atomic Mass: Lesson 3
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. It is 3 of 4 in the series titled "Atomic Mass."
Sophia Learning
Sophia: Atomic Mass: Lesson 1
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. It is 1 of 4 in the series titled "Atomic Mass."
Sophia Learning
Sophia: Atomic Mass: Lesson 2
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. It is 2 of 4 in the series titled "Atomic Mass."
Sophia Learning
Sophia: Atomic Mass: Lesson 4
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. Module includes a slideshow and a quiz.
CK-12 Foundation
Ck 12 Exploration Series: Simulations: Physics: Tire Pressure
[Free Registration/Login Required] Uncover the relationship between pressure, force, and contact area in the context of a fire truck. Play with vehicle mass, number of tires, and guage pressure with this simulation to see what...
CK-12 Foundation
Ck 12: The Mole Concept
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will identify three methods for measuring the amount of matter in a sample. They will define the mole and its relationship...
Other
Characteristics of Energy and Matter
A lengthy page from the Fundamentals of Physical Geography site. Energy is distinguished from matter, and the different forms of energy are identified and discussed. Four types of heat transfer (convection, advection, conduction,...
Dartmouth College
Dartmouth College: Chem Lab: Electrons in a Box
This site has an interactive component that allows you to change the mass, number of electrons and the length of the box and see how it affects the energy levels. Requires Java.
California State University
California State University: Proton, Electron, Neutron
An interesting and useful tool to practice recognition/calculation of atomic number, mass, and number of neutrons, electrons, etc. Can be used with all elements.