Hi, what do you want to do?
Other
The Acids, Alkalis and Salts Crossword
Test your chemistry knowledge with this exciting hands-on online crossword puzzle. Creative Chemistry has developed an on-line game for teachers to utilize in their classrooms.
Dartmouth College
Dartmouth College: Acids, Bases, and Buffers 1: Monoprotic and Polyprotic Acids
In this experiment, you will explore the behavior of the monoprotic acid (acetic acid) and the polyprotic acid (phosphoric acid). By titrating, you will examine the acid and conjugate base species present across the pH scale and the...
American Chemical Society
Middle School Chemistry: P H and Color Change
Students see a demonstration of a color change using universal pH indicator and then change the concentrations of an acid and a base using a universal indicator to test the pH of the resulting solutions. Through an animation, they see...
Cosmo Learning
Cosmo Learning: Senior Chemistry With Chemguy
A collection of video lectures from a general chemistry class for high school students. Lectures cover topics such as equilibrium, thermochemistry, electrochemistry, and acids and bases with forty-four lectures. Lectures vary in length...
University of Waterloo (Canada)
Univ. Of Waterloo: Conjugate Acid Base Pairs
This page, created by a chemistry professor from the University of Waterloo, provides an introduction to conjugate acid-base pairs.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Weak Acid Equilibrium
Learn about what happens when a weak acid is added to water. View examples, an acid dissociation constant table, and a study sheet.
Khan Academy
Khan Academy: Water Autoionization and Kw
Learn about the autoionization of water, the autoionization constant Kw, and the relationship between [H+] and [OH-] in aqueous solutions.
Sophia Learning
Sophia: Calculations: P H, P Oh, [H+], [Oh ]
Created to teach students of the 21st century, SOPHIA is bringing chemistry calculations straight to your fingertips. Become the commander of your own learning experiences as you take part in this interactive lesson.
PBS
Zoom: Kitchen Chemistry
Did you know that chemistry can be found in your kitchen? Enter this amazing virtual world where your first stop is to the kitchen. Locate the clues and move on to the Lab Journal where you can test each item in two experiments: Cabbage...
Chemistry Collective
Chem Collective: Virtual Lab
Perform virtual lab experiments safely. Pour and mix specific amounts of various solutions on screen and observe changes in temperature and pH. Choose glassware and other tools (ie. bunsen burner, scale) to assist with virtual lab...
Chemistry Collective
Chem Collective: Virtual Lab: Default Virtual Lab Stockroom
Perform virtual lab experiments safely. Pour and mix specific amounts of various solutions on screen and observe changes in temperature and pH. Choose glassware and other tools (ie. bunsen burner, scale) to assist with virtual lab...
Wolfram Research
Wolfram Science World: Organic Chemistry
This site from ScienceWorld provides a brief look at some of the more common organic functional groups. The carboxylic acid group is also given. Quite a few links are provided at the bottom of the page for additional information.
Science Buddies
Science Buddies: Using Pennies to Test How P H Affects Copper Corrosion
In this science fair project, use a color-based reaction to test how pH affects copper corrosion in pennies.
Chem Tutor
Chem Tutor: Chemistry: Titration and P H Math Problems
This is a titration and ph math problem exercise; the answers are also provided.
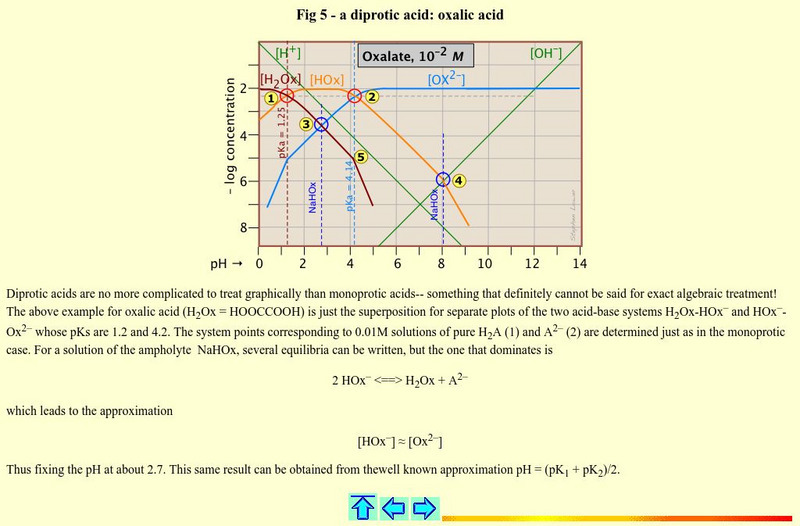
Simon Fraser University
Chem1 Virtual Textbook: Oxalic Acid
Acting as an overview from the General Chemistry Virtual Textbook, this site acts as one in a series of slides on acid-base systems. This particular slide deals with a diprotic acid, oxalic acid.
CK-12 Foundation
Ck 12: Acid Base Neutralization
[Free Registration/Login may be required to access all resource tools.] Students will understand and differentiate among acid-base reactions, precipitation reactions, and oxidation-reduction reactions.
TeachEngineering
Teach Engineering: Engineering Out of Harry Situations
Under the "The Science Behind Harry Potter" theme, a succession of diverse complex scientific topics are presented to students through direct immersive interaction. Student interest is piqued by the incorporation of popular culture into...
University of Arizona
Ua: Chemistry Tutorial
This general tutorial begins with an explanation of the polarity of the water molecule and the effects this polarity has on the properties of water. Goes on to introduce organic molecules and has a thourough tutorial on the third page.
Cosmo Learning
Cosmo Learning: Introduction to Solid State Chemistry
A collection of video lectures from a course that investigates the application of basic chemistry principals to engineering systems. The course draws from industrial practice to give examples of the application in engineering systems. In...
CK-12 Foundation
Ck 12: Chemistry for High School
This digital textbook covers core chemistry concepts and includes interactive features, real-world examples, and videos.
CK-12 Foundation
Ck 12: Physical Science: P H Concept
[Free Registration/Login may be required to access all resource tools.] Definition of pH and the strength of acids and bases. How to use the pH scale and why pH is important to living things.
Texas Instruments
Texas Instruments: Holt Modern Chemistry: Acid Base Titration
This activity Acid-Base Titration from the chapter Acid-Base Titration and pH uses the calculator program TITRAFC4 to display titration curves for the titration of a strong or weak acid with a strong base. The titration curve shows the...
Texas Instruments
Texas Instruments: Microscale Acid Base Titration
In this activity, students' will use a pH sensor to determine the change in pH during a titration of a known concentration of NaOH and unknown concentration of HCl. Students' will determine the concentration of unknown HCl.
US Environmental Protection Agency
Epa: Measuring P H Experiment
This is an activity that deals with acid rain. "This experiment will illustrate how to measure the approximate pH of chemicals in water using a pH indicator." It is easy to follow the comprehensive set of directions.









![Sophia: Calculations: P H, P Oh, [H+], [Oh ] Unit Plan Sophia: Calculations: P H, P Oh, [H+], [Oh ] Unit Plan](https://content.lessonplanet.com/knovation/original/218428-81e36f3b80bf76c44b558309eac1c9b2.jpg?1661242989)