University of St. Andrews (UK)
University of St. Andrews: Niels Henrik David Bohr
This resource presents a biography of Bohr, including many personal tidbits, as well as a substantial treatment of his scientific work. Several interesting quotes by and about Bohr are included. It also links to several other sources.
Boise State University
Boise State University: Atoms: A Virtual Field Trip Through Time and Space
Learn about the models of the atom that have been proposed throughout history. Presents Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern theory of the atom. Sections are accompanied by journal...
CK-12 Foundation
Ck 12: Atomic Theory
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will explain the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. They will also...
CK-12 Foundation
Ck 12: Chemistry: Bohr's Atomic Model
[Free Registration/Login may be required to access all resource tools.] Explains the basic principles of the Bohr hydrogen atom.
Simon Fraser University
Chem1 Virtual Textbook: Bohr's Theory
Acting as part of an overview on quantum theory, this section of the site answers the question, "How did Bohr's theory save the planetary model, for a while?" A section below also discusses the primary problems with Bohr's theory.
Vision Learning
Visionlearning: Atomic Theory: Bohr and the Beginnings of Quantum Theory
Description of the ideas and experimentation that led to quantum theory. Focus on electrically charged ions and isotopes.
Other
Siyavula Education: Everything Maths & Science: Models of the Atom
Discusses models of the atom developed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Includes clear illustrations and a short comprehension exercise at the end.
Texas Education Agency
Texas Gateway: Atoms, Elements and the Periodic Table: The Atomic Model [Pdf]
A slideshow looking at the contributions of scientists over time to our understanding of atomic theory. Looks at models of the atom developed by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick, as...
Other
Crocodile Clips: Absorb Chemistry: History of the Atom
A tutorial that presents models of the atom proposed by John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr. Each is supported by an animated illustration. Includes comprehension questions and a quiz at the end.
Tom Richey
Slide Share: Atomic Models: Everything You Need to Know
A detailed slideshow for Grade 8 students that looks at the history of our understanding of atoms. Looks at the ideas presented by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr, and the modern-day wave model. A...
Tom Richey
Slide Share: Atomic Structure
Slideshow looking at the history of models of the atom, including those proposed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Discusses subatomic particles, including the numbers of protons, neutrons,...
Concord Consortium
Concord Consortium: What Are Nature's Building Blocks?
Activity 4 What are the electrons? This question is answered in the context of Niels Bohr's model and the probability model of atomic structure.
Other
A Short History of the Atom
A classroom wiki where students present profiles of scientists who developed models of the atom or who contributed to the understanding of atomic theory. Covers Democritus, Aristotle, John Dalton, J.J. Thomson, Ernest Rutherford, Marie...
Other
Chemtopics: Development of Modern Atomic Theory [Pdf]
A summary of the achievements of J. J. Thomson, Ernest Rutherford, Niels Bohr, and Erwin Schrodinger.
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
Other
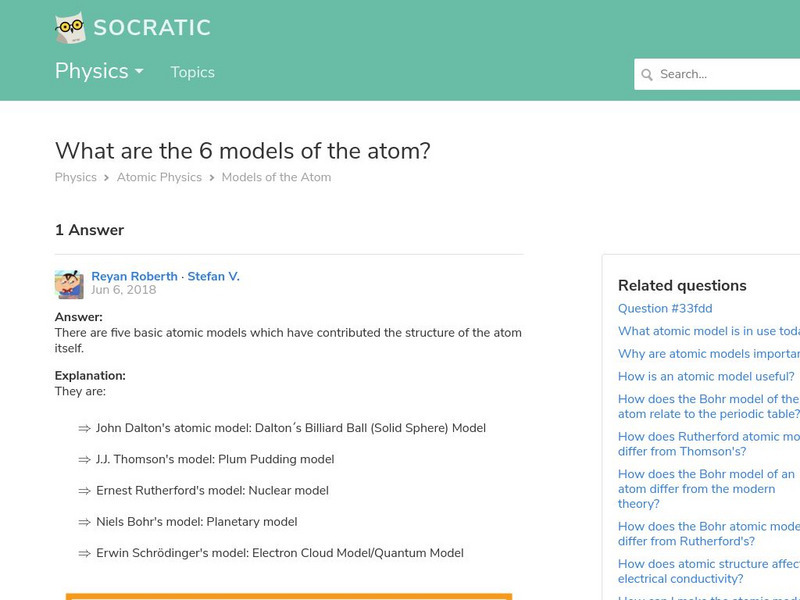
Socratic: What Are the 6 Models of the Atom?
Brief explanation of six major models of the atom along with illustrations. Covers the Greek model (Democritus), John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern electron cloud or quantum mechanics model.
Other
Holonity: The Atom From Solid Balls to Sparkling Ghosts
Presents the history of the atom with a New Age perspective on some aspects. It describes the concept of the atom in Vedic philosophy and that developed by Democritus. Goes on to look at the models proposed by John Dalton, J.J. Thomson,...
Other
Slide Player: History of the Atomic Model
Short slideshow looking at the history of models of the atom, including the contributions of Aristotle, Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr.
Simon Fraser University
Chem1 Virtual Textbook: Spectrum of a Guitar String
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses spectrum in relation to Bohr's model. Included in the topics covered are standing waves, boundary condition,...
Nobel Media AB
The Nobel Prize: Nobel Prize in Physics 1922 Presentation Speech
This Nobel Foundation site provides the "Presentation Speech by Professor S.A. Arrhenius, Chairman of the Nobel Committee for Physics of the Royal Swedish Academy of Sciences, on December 10, 1922."
PBS
Pbs Learning Media: That's My Theory!
Become a game show contestant in this online activity from A Science Odyssey and ask a series of questions to a panel of mystery scientists, using the answers to determine which scientist is Einstein.
Chem4kids
Chem4 Kids: Atoms
This site provides a detailed overview of atoms. Content explores an atom's structure, as well as what ions are, how atoms bond, what compounds are (including how to name compounds), and what isotopes are.