Sophia Learning
Sophia: Periodic Table Organization: Lesson 1
This lesson will explore the layout of the periodic table by defining different groups and categories of elements. It is 1 of 3 in the series titled "Periodic Table Organization."
Web Elements
Web Elements Periodic Table: Strontium
This site provides information about strontium and the reactions it has with water, air, halogens, acids, and bases.
Science Struck
Science Struck: Radium and Its Uses
Provides information about the discovery of radium, where it is found, its properties, and its uses.
Curated OER
Educational Technology Clearinghouse: Clip Art Etc: Sir Humphrey Davy
(1778-1829) British chemist and inventor remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine.
Soft Schools
Soft Schools: Periodic Table Groups Quiz
Take an interactive quiz over the groups on the periodic table of elements. After completing the quiz, check your score, and then revisit any incorrect question for further review.
Science4Fun
Science4 Fun: Magnesium
Fun and interesting information about magnesium, the 18th most abundant element in the universe. Learn about its characteristics, uses, where it is found, and discovery.
Web Elements
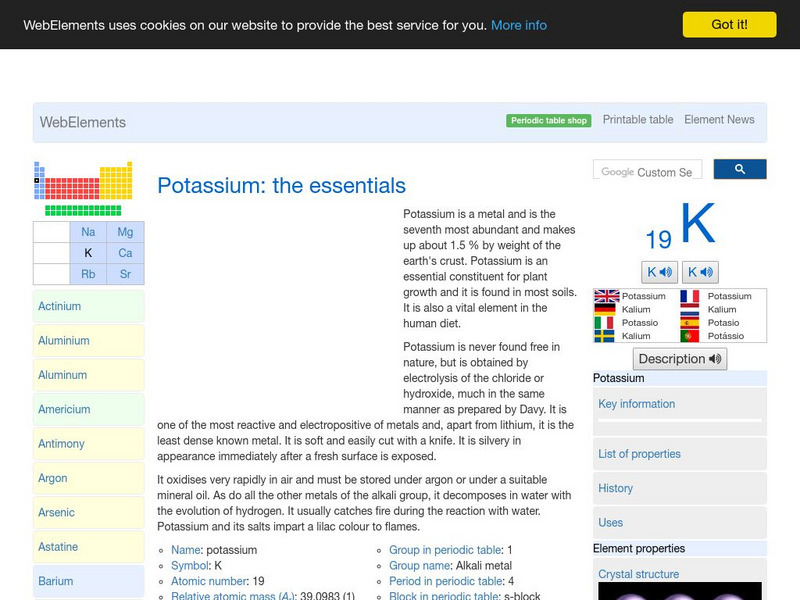
Web Elements Periodic Table: Potassium
This WebElements site provides a great deal of elemental information. There are audio files demonstrating the correct pronunciation of the element name, pictures showing the electron shells, and information on isotopes, properties and more.
Other
Chemical Elements: Potassium
A nice, clear site, containing a good deal of elemental information, including atomic weight, density, boiling point, isotopes and more. An image shows the electron energy levels for the element.