Curated OER
Silica Tetrahedron Model
Very simply, pairs of learners construct a model of the tetrahedral silica structure using raisins and toothpicks. They dip it into a soapy solution and then blow a bubble "atom" into its center. The lesson plan gives instructions that...
Virginia Department of Education
Historical Models of Atoms
What does the past have to do with today? Young scientists find that answer as they learn more about past chemists and their significant contributions to the field. Pupils use the Internet to research historical figures and create a...
Virginia Department of Education
Average Atomic Masses
Facilitate learning by using small objects to teach the principles of atomic mass in your science class. Pupils determine the average mass of varying beans as they perform a series of competitive experiments. They gather data and...
Royal Society of Chemistry
Complex Ion Shapes
Things are really shaping up! Provide young chemists with polyatomic ion practice using an interesting interactive. Individuals complete puzzles focused on molecular geometry and complex ions.
National Institute of Open Schooling
Atomic Structure
Learners explain historical findings such as Rutherford and Bohr's contributions, explain wave particle duality, and formulate Heinsenberg's uncertainty principle. They also draw s, p, and d orbitals, explain more historical findings,...
Concord Consortium
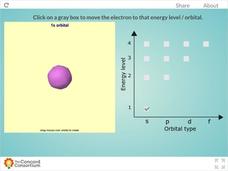
Energy Levels of a Hydrogen Atom
Tired of blowing up countless balloons to illustrate orbital shapes around an atom? Give your lungs a break and use an interactive instead! Learners observe s, p, d, and f orbitals through the first four energy levels using hydrogen as a...
Curated OER
Typical Numeric Questions for Physics I - Atomic Spectra
Seven practice problems are presented to physics pros in this assignment. Given the wavelengths, they perform computations for emission spectra. This brief worksheet makes an appropriate pop quiz.
Curated OER
VSEPR And Polarity
For this VSEPR theory worksheet, students evaluate the electron-pair geometry of organic and inorganic molecules. They construct Lewis structures and resonance structures for 17 compounds and complete 3 short answer questions.
Curated OER
Atom Basics Test
Simple in format and standard in content, this resource is an assessment of your beginning chemists' grasp of the atom. Using a periodic table of elements, they fill in a chart of missing chemical formulas, atomic masses, and numbers of...
Curated OER
Carbon Bonds in Chemistry
Venture into the world of macromolecules with three exciting, distinct laboratory activities. Young chemists examine the forms of carbon and discover how they are associated with atomic arrangement, construct models of carbon-containing...
Curated OER
Atoms, Molecules, and Chemical Bonds
In this atoms instructional activity, students review the parts of an atom, Bohr diagram, atomic number, mass number, and covalent bonds. This instructional activity has 5 drawings and 26 fill in the blank questions.
Curated OER
Atoms, Elements, Molecules, and Compounds
In this elements instructional activity, students review the Bohr model and define negative and positive subatomic particles. Students compare molecules and compounds. This instructional activity has 11 short answer questions and 4...
Curated OER
Biochemistry Assignment
In this biochemistry worksheet, students complete a table by filling in the missing information about different elements. Students draw the Bohr diagram and the Lewis dot diagram for several atoms.
Science Geek
Stoichiometry
Watch your class react when you tell them they're going to study stoichiometry! The lesson demonstrates four examples. Scholars must write and balance the chemical equation and then make the conversion to find the correct mass or volume....
Curated OER
How do protons stick together in a nucleus?
Students explain that the Standard Model of the atom includes particles beyond protons, neutrons, and electrons. They describe the nucleus as conglomeration of quarks that manifest themselves as protons and neutrons.
Cornell University
Electroplating
Silver pennies and copper nickels are made possible by applying some chemistry. Learners use electrolysis to coat a penny with zinc sulfate and a nickel with copper sulfate. Their investigation builds an understanding of electroplating...
Virginia Department of Education
Solution Concentrations
What happens when you combine 6.022 times 10 to the 23 piles of dirt into one? You make a mountain out of a mole hill. Scholars use dehydration to obtain percent composition and then calculate the molarity of the original solution.
Curated OER
The Physical Setting
Learners study atoms and their protons and neutrons. In this physical setting lesson students work together to complete a lab activity.
Curated OER
What do Atoms Look Like?
In this atom learning exercise, high schoolers answer 31 multiple choice questions about the structure of atoms, the periodic table, the reactivity of elements, orbital diagrams and the families of elements.
Curated OER
Introduction to Atoms
In this atoms activity, students answer four different sets of questions related to atoms (fill in the blank, multiple choice, word puzzle and true and false).
Curated OER
Cell physiology and chemistry
Students design an experiment to discriminate between chemical diffusion, osmosis, facilitated diffusion and active transport through a membrane. Be specific about predictions and interpretations!
Curated OER
Pauli's Magical Water
Learners predict the shape of molecules using VSEPR theory. For this chemistry lesson, students differentiate a polar and nonpolar molecule. They discuss why water's polarity is very important.