Hi, what do you want to do?
Curated OER
Grating Spectrometer
Students calculate the Balmer series. In this physics lesson, students observe hydrogen lamp spectra using spectrometers. They calculate wavelength and compare them with their theoretical calculations.
Georgia State University
Georgia State University: Hyper Physics: Hydrogen Energies and Spectrum
This site from Georgia State University gives information on the transitions of electrons between energy levels. The energy levels for electrons in the hydrogen atom are discussed. The Rydberg equation is stated and electron transitions...
University of Colorado
University of Colorado: Physics 2000: Balmer's Formula
A short description of a physics lab involving the determination of the wavelength of the four spectral lines in the hydrogen emission spectrum. Perhaps the most important part of the page is the picture of the spectrometer.
Simon Fraser University
Chem1 Virtual Textbook: Spectrum of the Hydrogen Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the hydrogen atom and its relation to spectrum. Included in the discussion is information on the Bohr model...
Friesian School
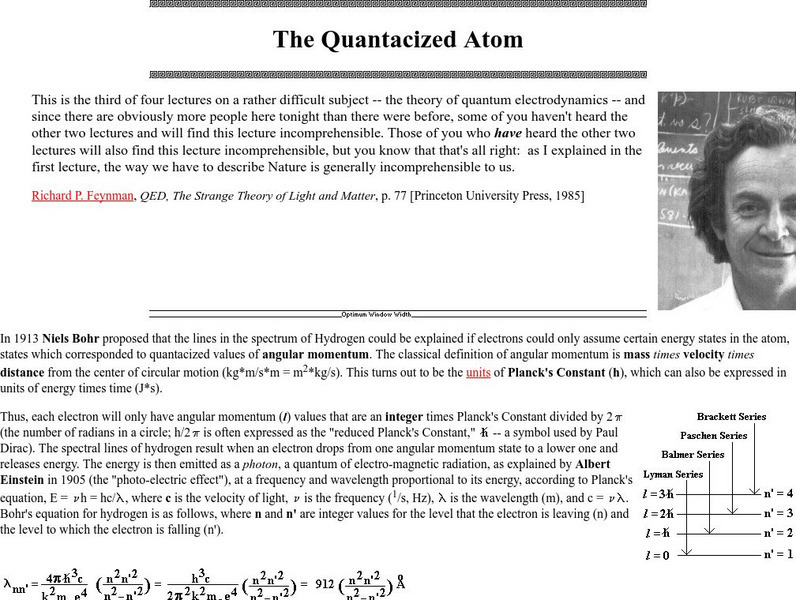
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Other
Brockport High School: Energy Levels of Hydrogen Atom
From the Brockport High School Physics Labs web pages. Includes an excellent graphic depicting the energy levels of a hydrogen atoms and portraying the electron level transitions for the Lyman, Balmer, and Paschen series. Includes both...
Other
Fact index.com: Balmer Series
Fact-Index.com offers a brief dictionary definition of the Balmer series, including hyperlinked terms.
Other
Fact index.com: Paschen Series
Fact-Index.com offers a dictionary definition of the Paschen series, including hyperlinked terms.
Other
Ap Physics Lab: Energy Levels of the Hydrogen Atom
A lab activity from an AP Physics course. Students measure the energy changes associated with electron level transitions to the second energy level for hydrogen gas. Includes directions and suggestions. Ideal for a student project or lab...
University of St. Andrews (UK)
University of St. Andrews: Johann Jakob Balmer
Describes the life and scientific contributions of Johann Jakob Balmer. Discussion focuses on Balmer's contributions to the line spectra of hydrogen.