Khan Academy
Khan Academy: Arrhenius Definition of Acids and Bases
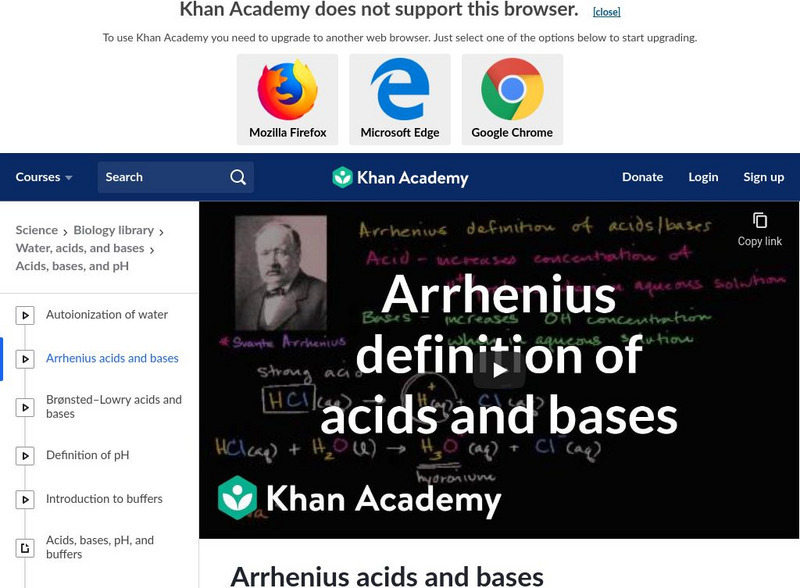
Arrhenius definition of acids and bases. [7:50]
Khan Academy
Khan Academy: Arrhenius Equation
Definition of rate constant k, the pre-exponential factor A, and activation energy are introduced. Understand how the exponential part of the Arrhenius equation depends on activation energy and temperature. [9:21]
Khan Academy
Khan Academy: Forms of the Arrhenius Equation
Know how to write different forms of the Arrhenius equation. Use the Arrhenius equation to look at how changing temperature and activation energy affects collisions. [6:36]
Khan Academy
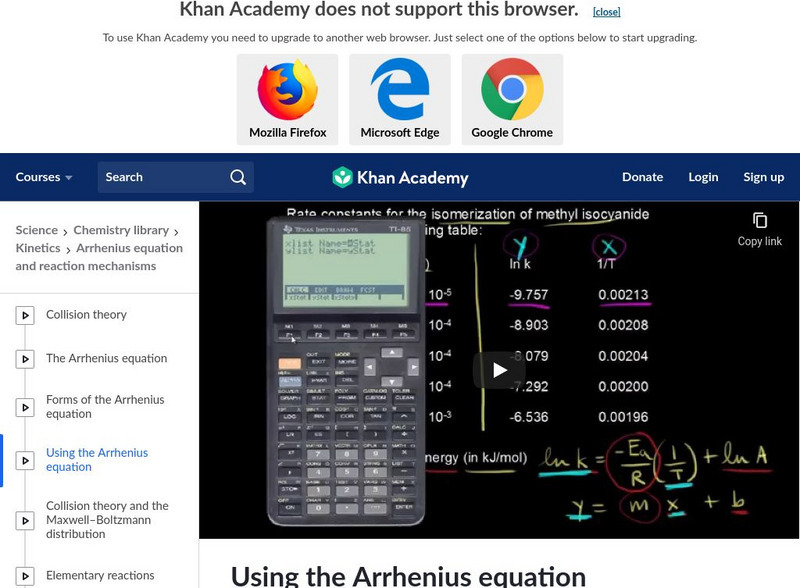
Khan Academy: Using the Arrhenius Equation
Know how to use the Arrhenius equation to calculate the activation energy. [11:01]
Khan Academy
Khan Academy: Biology: Arrhenius Definition of Acids and Bases
Learn the Arrhenius'definitionon of acids and bases in this video. [7:49]
Khan Academy
Khan Academy: Chemistry: Acid Base Introduction
The video explores how Arrhenius, Bronsted Lowry and Lewis define acids and bases. Also understand how ions disassociates in water through examples of chemical reactions. [18:37]
Sophia Learning
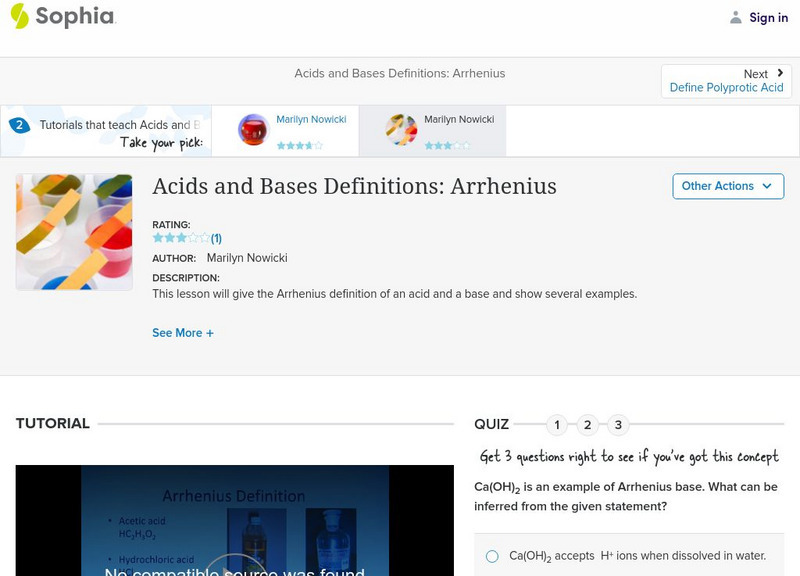
Sophia: Acids and Bases Definitions: Arrhenius: Lesson 1
This lesson will give the Arrhenius definition of an acid and a base and show several examples. It is 1 of 2 in the series titled "Acids and Bases Definitions: Arrhenius."
Khan Academy
Khan Academy: Arrhenius Definition of Acids and Bases
A video over viewing the Arrhenius definition of acids and bases. [7:49]
Khan Academy
Khan Academy: Using the Arrhenius Equation
See examples of how to calculate activation energy by using the Arrhenius equation. [11:06]
Khan Academy
Khan Academy: Arrhenius Equation
The Arrhenius equation is k = Ae^(-Ea/RT), where A is the frequency or pre-exponential factor and e^(-Ea/RT) is the fraction of collisions that have enough energy to react (i.e., have energy greater than or equal to the activation energy...
Sophia Learning
Sophia: Acids and Bases Definitions: Arrhenius: Lesson 2
This lesson will give the Arrhenius definition of an acid and a base and show several examples. It is 2 of 2 in the series titled "Acids and Bases Definitions: Arrhenius."