Khan Academy
Khan Academy: Emission Spectrum of Hydrogen
An explanation of solving for proton energy using the Balmer-Rydberg equation with a focus on hydrogen. [10:50]
Bozeman Science
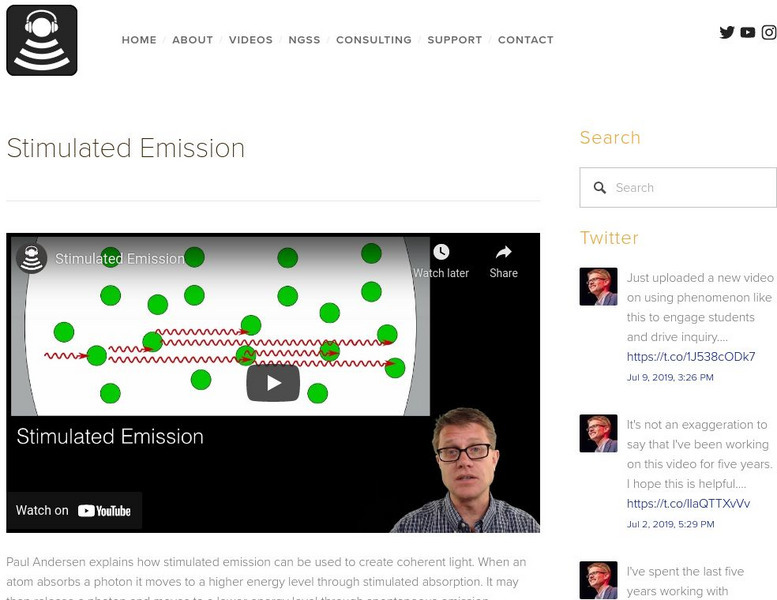
Bozeman Science: Stimulated Emission
In the following video Paul Andersen explains how stimulated emission can be used to create coherent light. When an atom absorbs a photon it moves to a higher energy level through stimulated absorption. It may then release a photon and...