Vision Learning
Visionlearning: Chemistry: The Nature of the Chemical Bond
This site talks about ionic, polar covalent, and non-polar covalent bonding.
American Chemical Society
Middle School Chemistry: Chapter 4: The Periodic Table and Bonding
Six middle school chemistry lessons about the periodic table and bonding complete with handouts and animations.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Molecular Structure: Audio Book
This audio book, narrated by Mark Bishop, describes the formation of covalent bonds. Lewis dot structures help highlight the valence electrons used to determine which bonds form. Also find links to animations and tutorials about other...
Texas Instruments
Texas Instruments: Covalent Bonding
This activity helps students review basic covalent nomenclature.
Science Education Resource Center at Carleton College
Serc: Loopy Lewis Dot Diagrams
Students will use colored fruit loops to organize valence electrons to develop and master the basics of Lewis Dot diagrams. They will develop processing and critical thinking skills and also master a model of bonding.
Science Education Resource Center at Carleton College
Serc: Valence Electrons and Trends in the Periodic Table
This instructor led activity will produce a partially filled periodic table that contains electron-dot models for the first twenty elements in the appropriate boxes. It will be used as a visual tool for young scholars to connect concepts...
Ohio State University
Ohio State University: Electronegativity & Bond Polarity
Excellent graphics help this page explain the relationship between electronegativity and bond polarity.
Texas Education Agency
Texas Gateway: Covalent Bonding: Electron Dot Diagrams
Given descriptions, diagrams, scenarios, or chemical symbols, students will model covalent bonds using electron dot formula (Lewis structures).
Other
University of Texas at Dallas: Attractions in Compounds
Explanation of attractive forces and energy changes involved in bonding.
BBC
Bbc: Gcse Bitesize: Bonding: Covalent Bonds
This section of the lesson module focuses on covalent bonds, which are formed between non-metal atoms that share a pair of electrons. It includes links to a video and a test. Other types of chemical bonds are covered elsewhere in the...
Frostburg State University
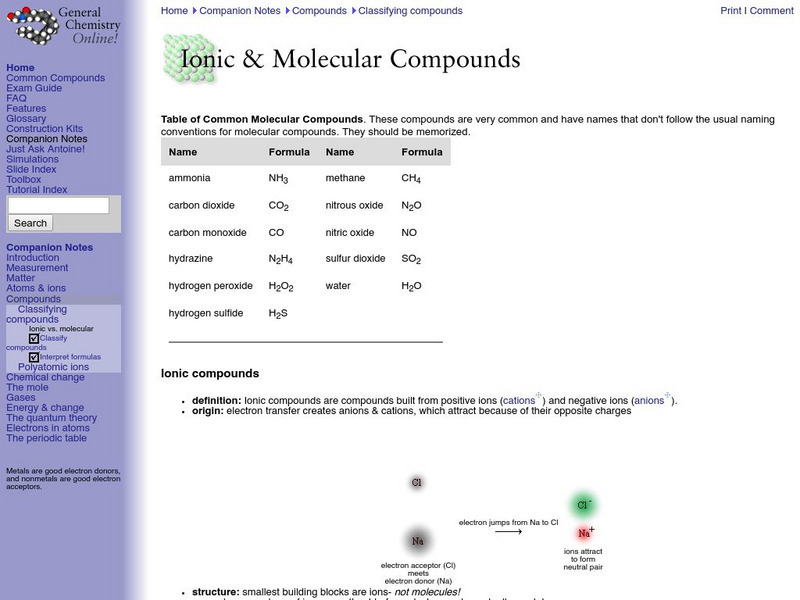
General Chemistry Online: Ionic and Molecular Compounds
Provides a good outline of the concepts involved in ionic and covalent bonding, with links to definition of terms. Features a list of common molecular compounds and a chart that compares ionic and molecular compounds.
CK-12 Foundation
Ck 12: Chemistry: Covalent Bonding
A learning module giving the definition of a covalent bond, the compounds and elements they make up and why they form. Module includes reading and review questions.
CK-12 Foundation
Ck 12: Physical Science: Covalent Bonding
[Free Registration/Login may be required to access all resource tools.] Definition of covalent bond, the compounds and elements they make up, and why they form.
Utah Education Network
Uen: Grouping Bonds
Students will group a selection of electron dot structures and determine similarities and differences between covalent and ionic bonding.
CK-12 Foundation
Ck 12: Chemistry for High School
This digital textbook covers core chemistry concepts and includes interactive features, real-world examples, and videos.
McMaster University
Mc Master University: Molecular Structure
This PowerPoint presentation features 33 slides that explain chemical bonding and molecular shape.
Other
Personal Site: Covalent Bonding Single Bonds
This site is a personal site that gives "a simple view of covalent bonding" including explanations, diagrams, and examples.
Sophia Learning
Sophia: Characteristics of Chemical Bonds: Lesson 2
This lesson will present the basic properties and characteristics of chemical bonds. It is 2 of 4 in the series titled "Characteristics of Chemical Bonds."
Sophia Learning
Sophia: Characteristics of Chemical Bonds: Lesson 4
This lesson will present the basic properties and characteristics of chemical bonds. It is 4 of 4 in the series titled "Characteristics of Chemical Bonds."
Oklahoma State University
Oklahoma State University: Bonding Rules of Thumb
Simple rules to help students identify types of chemical bonding.
McMaster University
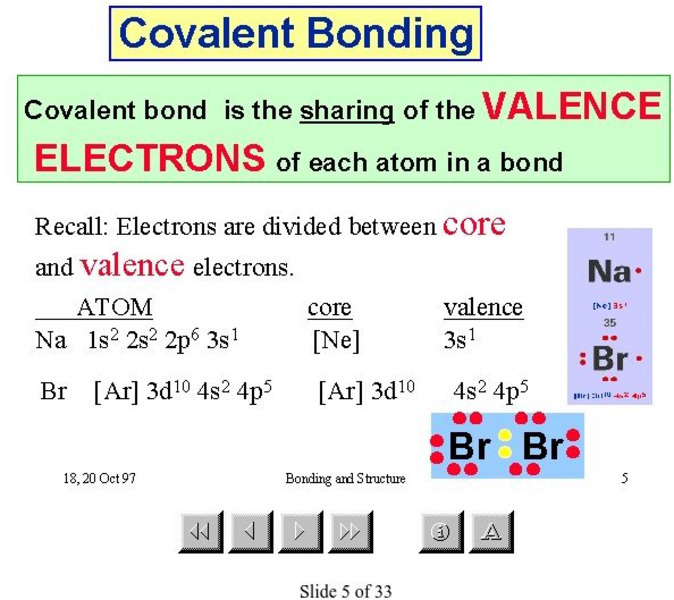
Mc Master University: Covalent Bonding
Slides 5 through 8 in this presentation from the McMaster University explain covalent bonding.
Other
University of Texas Dallas: Electron Appetites
The first section of this lecture on polarity and electronegativity discusses what polar covalent bonds are by using carbon dioxide as an example.
Curated OER
Simon Fraser University: All About Chemical Bonds
Irving Langmuir (1881-1967), chemist who won the Nobel Prize in 1932 for work on the chemistry of surfaces and monomolecular layers, and worked on the theory of electron pair bonding following N.G. Lewis.