Khan Academy
Khan Academy: Nickel Cadmium Battery
Know how to balance the redox reaction (in base) that occurs in a nickel-cadmium battery. [11:03]
Khan Academy
Khan Academy: Standard Reduction Potentials
Know how to use a table of standard reduction potentials to calculate standard cell potential. Identify trends in oxidizing and reducing agent strength. [9:03]
Khan Academy
Khan Academy: Using Reduction Potentials
Find an example of using the table of standard reduction potentials to calculate standard cell potential. [5:58]
Khan Academy
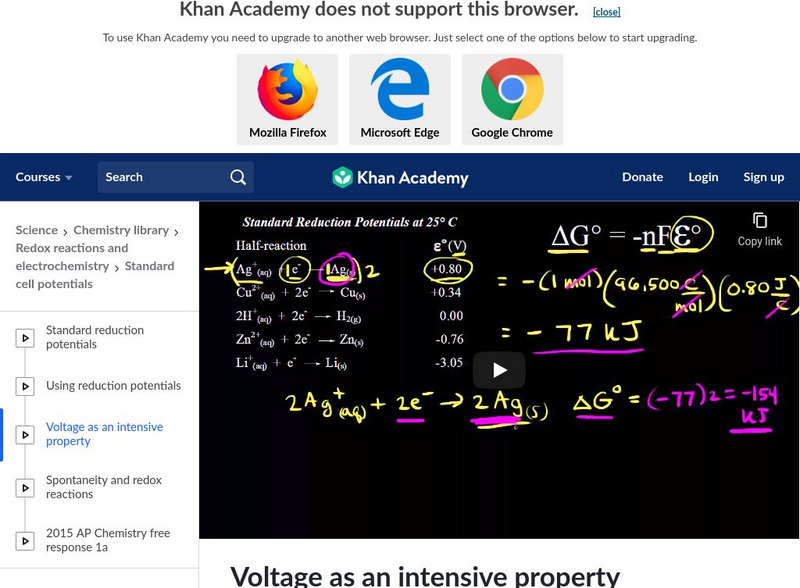
Khan Academy: Voltage as an Intensive Property
A demonstration that voltage is an intensive property by calculating the voltage and change in Gibbs free energy for a half reaction. [5:16]
Khan Academy
Khan Academy: Spontaneity and Redox Reactions
Investigate how to use standard cell potential to predict whether a redox reaction will be spontaneous under standard conditions. [12:17]
Khan Academy
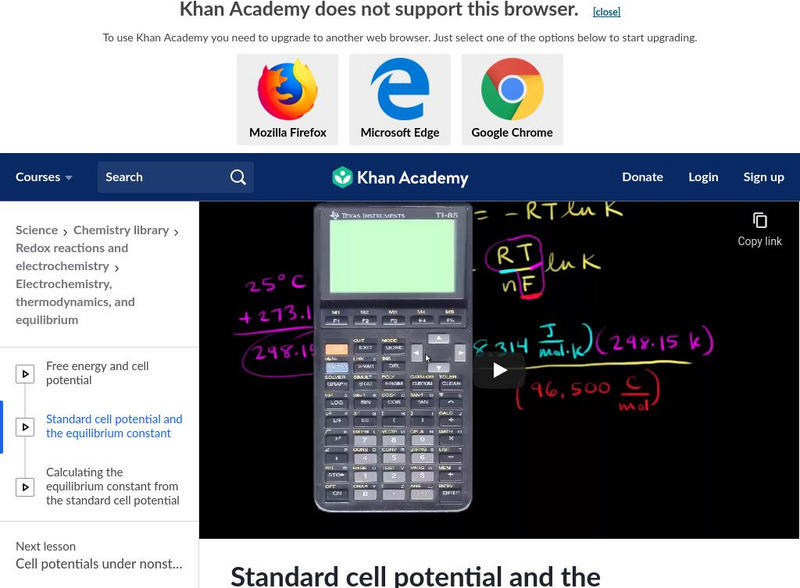
Khan Academy: Standard Cell Potential and the Equilibrium Constant
Find the relationship between standard cell potential and equilibrium constant K. [5:18]
Khan Academy
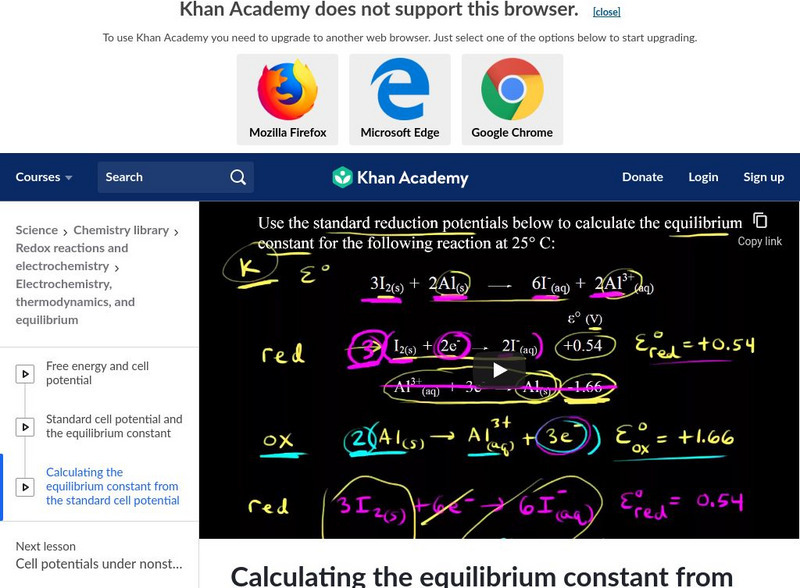
Khan Academy: Calculate the Equilibrium Constant From Standard Cell Potential
Walk through an example problem for calculating the equilibrium constant K using the standard cell potential. [9:37]
Khan Academy
Khan Academy: Galvanic Cells and Changes in Free Energy
Recognize the relationship between Gibbs free energy, reaction quotient Q, and cell voltage. [12:31]
Khan Academy
Khan Academy: Nernst Equation
Derive a few different forms of the Nernst equation, the relationship between Gibbs free energy and reaction quotient Q. [6:29]
Khan Academy
Khan Academy: Using the Nernst Equation
Use the Nernst equation to calculate the cell potential when concentrations are not standard conditions. [11:24]
Khan Academy
Khan Academy: Concentration Cell
Use the Nernst equation to calculate cell potential for a concentration cell. [9:32]
Khan Academy
Khan Academy: Introduction to Electrolysis
This video compares a voltaic cell to an electrolytic cell. [6:51]
Khan Academy
Khan Academy: Quantitative Electrolysis
Calculate how much zinc deposits on the zinc electrode after 1.0 h when a current of 5.0 A is applied to the battery. [6:54]
Khan Academy
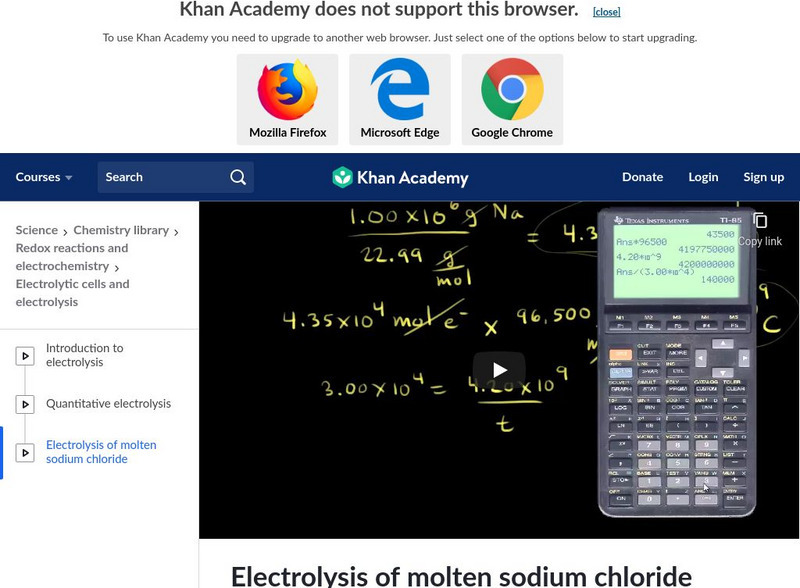
Khan Academy: Electrolysis of Molten Sodium Chloride
Understand an example of a quantitative electrolysis problem using molten sodium chloride. [12:20]
Khan Academy
Khan Academy: Rate of Reaction
Definition of reaction rate, and examples of calculating the average rate of reaction. [9:09]
Khan Academy
Khan Academy: Finding Units of Rate Constant K
Discover how to find the units for the rate constant k for a zero, first, or second order reaction. [5:05]
Khan Academy
Khan Academy: Experimental Determination of Rate Laws
Understand this example using initial rates to find the order in each reactant, the overall order, and the rate constant k. [12:21]
Khan Academy
Khan Academy: First Order Reaction (With Calculus)
Deriving the integrated rate law for first-order reactions using calculus. How you can graph first-order rate data to see a linear relationship. [7:07]
Khan Academy
Khan Academy: Plotting Data for a First Order Reaction
Identify an example of graphing first-order rate data to see a linear relationship and calculating rate constant k from the slope. [9:13]
Khan Academy
Khan Academy: Half Life of a First Order Reaction
Understand how to derive the half-life equation of a first-order reaction starting from the integrated rate law. [8:12]
Khan Academy
Khan Academy: First Order Reaction Example
An example of how to use the integrated rate law to solve for time and concentration. Understand how to calculate the half-life for a first-order reaction. [10:15]
Khan Academy
Khan Academy: Rate Constant K From Half Life Example
An example problem shows how to find the rate constant k from the half-life of a first-order reaction. [6:10]
Khan Academy
Khan Academy: Second Order Reaction (With Calculus)
Understand the process of deriving the integrated rate law for second order reactions using calculus. Know how to graph second order rate data to see a linear relationship. [7:09]
Khan Academy
Khan Academy: Plotting Data for a Second Order Reaction
An example of graphing second order rate data to see a linear relationship, and calculating rate constant k from the slope is introduced from Khan Academy. [8:02]