Hi, what do you want to do?
Crash Course
3D Structure and Bonding - Crash Course Organic Chemistry
The organic molecules that make up life on Earth are more than just the 2-D structures we’ve been drawing so far. Molecules have 3-D shapes that help us understand what they can do. In this episode of Crash Course Organic Chemistry,...
Curated Video
Sp Hybridization in Alkynes: Exploring Triple Bonds
In alkynes, such as acetylene (C₂H₂), carbon atoms undergo sp hybridization. This involves the mixing of one 2s orbital and one 2p orbital, forming two sp hybrid orbitals. These orbitals align in a linear arrangement with a bond angle of...
Professor Dave Explains
Limitations of VSEPR Theory
We've learned about VSEPR theory, and we know how to use it to predict molecular geometry for a variety of organic molecules. But in fact, there are situations where predictions made with VSEPR theory do not line up with experimental...
msvgo
Bonding in Coordination Compounds
It explains bonding, properties & limitations of Valence bond theory & crystal field theory of coordination compounds.
Khan Academy
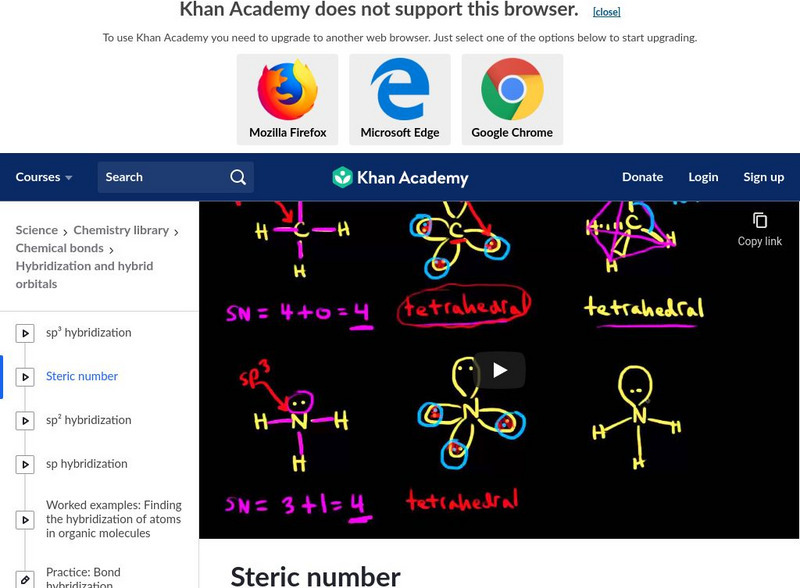
Khan Academy: Steric Number and Sp3 Hybridization
Find out how to use steric number to calculate the number of hybrid orbitals. [9:47]
Khan Academy
Khan Academy: Hybrid Orbitals: Tetrahedral Bond Angle Proof
Presents a three-dimensional model of a methane molecule, with its tetrahedral bond, to make it easier to see the structure and bond angle. [5:05]
Khan Academy
Khan Academy: Hybrid Orbitals: Sp2 Hybrid Orbitals
A video lecture exploring how to determine the amount of hybridization of a molecule. Video contains examples. [11:29]
Khan Academy
Khan Academy: Sp3 Hybridization
Understand sp3 hybrid orbitals and properties of sigma bonds. [10:43]