Hi, what do you want to do?
Nobel Media AB
The Nobel Prize: The Nobel Prize in Physics 1935 Presentation Speech
The Nobel Physics Chairman made this speech when presenting the Prize to Chadwick. It clearly explains the importance and depth of Chadwick's work. Site by Nobel e-Museum.
Concord Consortium
Concord Consortium: Stem Resources: How Electrons Move
A collection of interactive activities and games to explore how electric fields and magnetic fields move electrons and charged particles in directions that can be planned. Understand that knowing how to control the movement of electrons...
Georgia State University
Georgia State University: Hyper Physics: Hund's Rules
A complete overview of Hund's rules including exceptions.
Georgia State University
Georgia State University: Hyper Physics: Hund's Rules
An excellent overview of Hund's Rules. Discusses all three rules thoroughly with provided diagrams.
Georgia State University
Georgia State University: Hyper Physics: Relativistic Energy
A mathematically understandable presentation of relativistic energy. Parts include Relativistic Energy, Rest Mass Energy, Conservation of Energy, Pair Production, Relativistic Kinetic Energy, and even Kinetic Energy. A couple of...
California State University
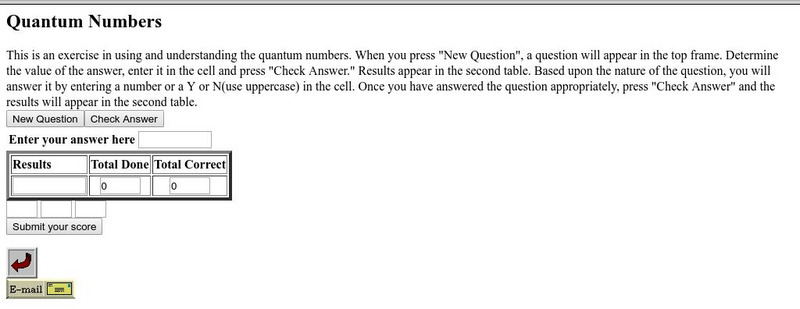
Csudh Project for Chemistry: Quantum Numbers
Use this exercise to review the use of and understanding of quantum numbers.
Oklahoma State University
Osu: Chemical Bonding Content in a Nutshell
An overview of chemical bonding and how valence electrons play a major role in these processes.
University of Colorado
University of Colorado: Physics 2000: Quantum Atom: Angular Momentum of an Electron
A very technical explanation of the angular momentum of an electron.
Science Education Resource Center at Carleton College
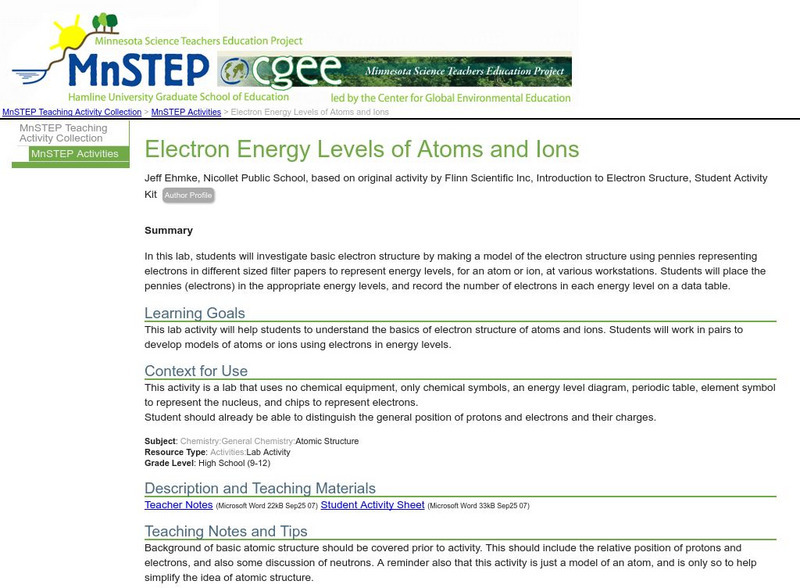
Serc: Electron Energy Levels of Atoms and Ions
In this lab, students investigate basic electron structure by making a model using pennies and different sized filter papers.
Science Education Resource Center at Carleton College
Serc: Tic Tac Toe Pick 3 in a Row Atomic Model Assignment
A choice menu assignment where students select between several extension activities that help understand the structure of the atom.
Science Education Resource Center at Carleton College
Serc: Drawing Atoms
This activity serves as an introduction to chemistry, and can be used to help students draw a two dimensional image of the atom.
Simon Fraser University
Chem1 Virtual Textbook: Electron Electron Repulsion
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses repulsion of electron pairs. Diagrams and charts of the repulsion are also provided.
Simon Fraser University
Chem1 Virtual Textbook: The Shell Model of the Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
Other
University of Winnipeg: The Pauli Exclusion Principle
This site provides information concerning the idea behind the Pauli Exclusion Principle. Includes basic terminology and explanations for understanding.
Purdue University
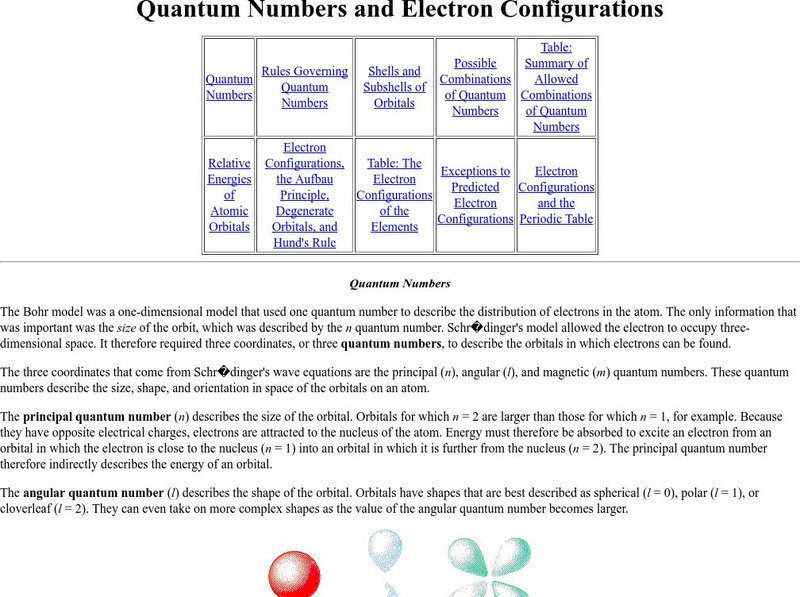
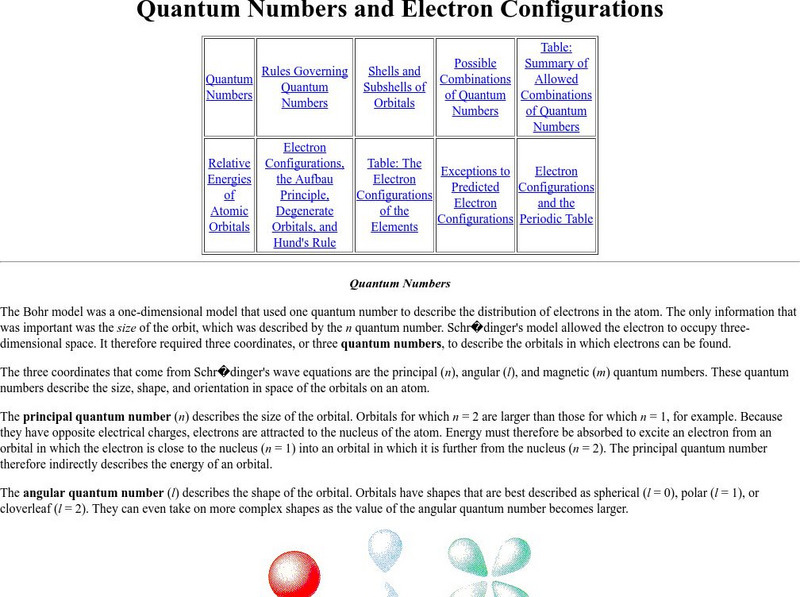
Purdue University: Quantum Numbers & Electron Configurations
This site from the Purdue University provides quantum numbers explained in detail and shown how they specify the atomic orbital of each electron and their energy levels. Electronic configurations are explained. Learning exercises with...
Purdue University
Purdue University: Orbital Shells
This site from the Purdue University provides an overview of atomic orbitals and how quantum numbers specify these orbitals. Includes a model that can be rotated and examined in 3D. Includes learning exercises and answers.
Purdue University
Purdue University: The Aufbau Principle
At this site from Purdue University, Electron Configurations, the Aufbau Principle, Degenerate Orbitals, and Hund's Rule are detailed. Includes learning exercises and answers.
Estrella Mountain Community College
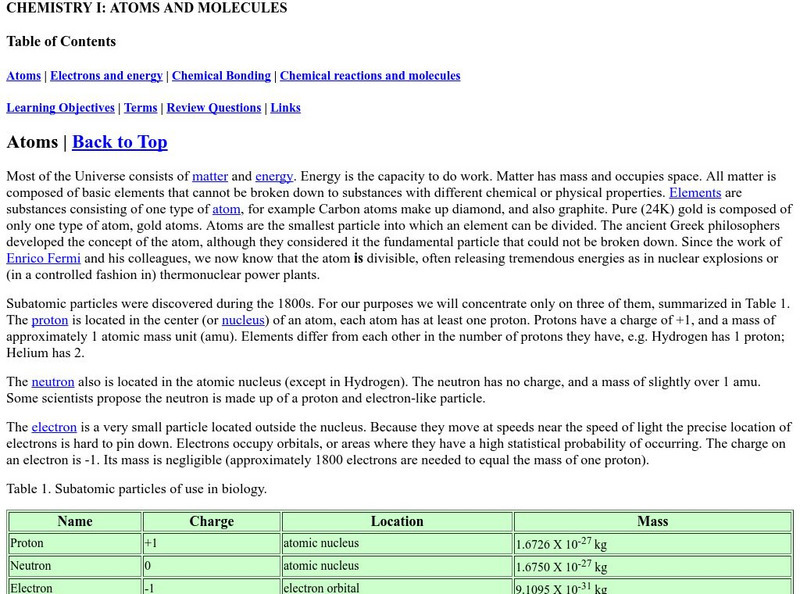
Online Biology Book: Chemistry I: Atoms and Molecules
In this online biology textbook, learn about atoms and molecules as they relate to life. Find out about topics such as electrons and energy, chemical bonding, and chemical reactions.
Crescent Public Schools
The Internet Science Room: The Periodic Table and Atomic Theory of Matter
A chemistry tutorial highlighting historical development of the atomic theory of matter and the periodic table of elements.
Crescent Public Schools
The Internet Science Room: Chemical Families
Students can use this chemistry tutorial to further their understanding of the families of elements on the Periodic Table of Elements.
Crescent Public Schools
The Internet Science Room: Oxidation Numbers and Chemical Bonding
Through animated diagrams and illustrated examples, students can further their understanding of chemical bonds and oxidation numbers.
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Electrochemistry
This online textbook chapter provides learners with advanced-level material on electrochemistry.
Michael Blaber, PhD
Fsu: Basic Concepts of Chemical Bonding: Exceptions to the Octet Rule
Lists the exceptions to the octet rule and provides a discussion and diagrams explaining each one. Includes clear diagrams illustrating this concept.
Michael Blaber, PhD
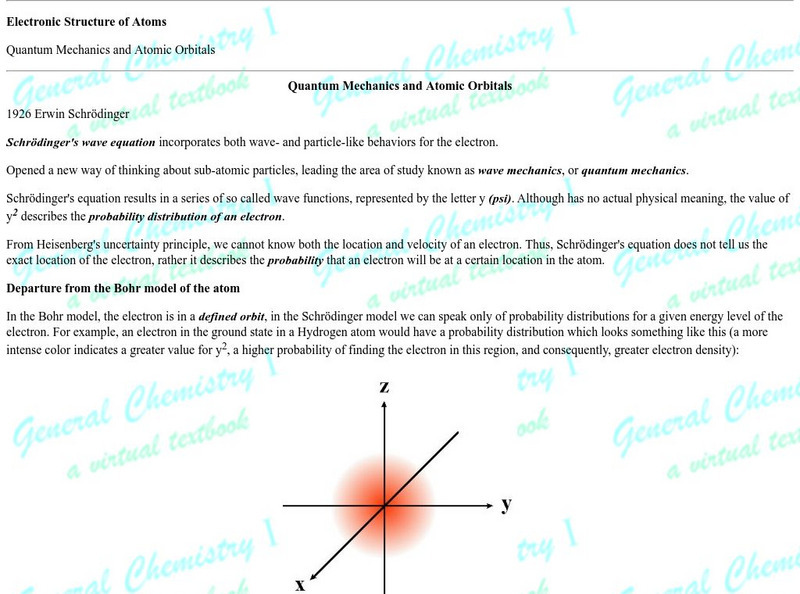
Fsu: Electronic Structure of Atoms: Quantum Mechanics & Atomic Orbitals
This article is provided by Florida State University. Schroedinger wave equation is explained and how quantum numbers are used to describe the energy level of any electron in an atom. The relationship of atomic orbitals to quantum...