Fuse School
Decomposition of Hydrogen Peroxide
Break down a great lesson on decomposition reaction with an informative video. Fourth in a 35-part series, the resource builds from the previous lesson explaining the role of a catalyst. The detailed video explains the experimental...
Fuse School
What Are Catalysts?
Investigate the power of a catalyst during a chemical reaction. The third video in a sizzling chemistry series explains the role of a catalyst in lowering the activation energy required in a chemical reaction. The narrator shares...
Ricochet Science
Enzymes: The Induced Fit Model
What occurs once a substrate binds to a protein? High schoolers view a short animation to witness the action of substrate binding and the resulting changes that occur in the protein. They conclude the video with a short discussion...
Berkeley University of California
Catalysis
Jump-start your understanding of catalysis in chemical reactions. An educational video explains the basic concept of catalysis and its relation to energy. It also introduces platinum plates as a catalyst for the reaction between...
Ricochet Science
How Enzymes Work
Has your class ever wondered exactly what jump starts chemical reactions within the body? Pupils view a short video segment that animates a chemical reaction, complete with enzymes and substrates, so they may further understand this...
Educreations
Le Chatelier's Principle
Equilibrium reactions are able to reach a steady state of products and reactants, but what happens when this careful balance is disturbed? With the help of this instructional video, young chemists learn to apply Le Chatelier's...
Curated OER
Oozing Pumpkin - Sick Science! #060
Make a Halloween pumpkin foam at the mouth! Using hydrogen peroxide, toothpaste, and yeast, you can recreate this chemical reaction in your classroom. It can lead to a discussion of the way ingredients mix together to make a new...
Steve Spangler Science
Patriotic Monster Foam - Cool Science Experiment
In honor of the 4th of July, Steve Spangler shows how to make an explosive concoction in red, white, and blue. Using hydrogen peroxide, dishwashing liquid, and yeast, he creates a colorful chemical reaction. Consider this combination...
Curated OER
Monster Foam
In honor of the 4th of July, Steve Spangler shows how to make an explosive concoction in red, white, and blue. Using hydrogen peroxide, dishwashing liquid, and yeast, he creates a colorful chemical reaction. Consider this combination...
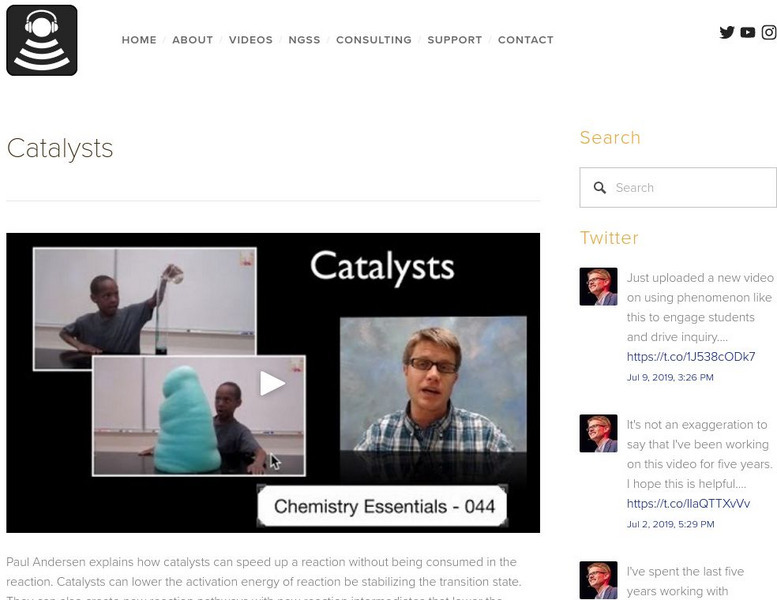
Bozeman Science
Bozeman Science: Catalysts
Paul Andersen explains how catalysts can speed up a reaction without being consumed in the reaction. Catalysts can lower the activation energy of reaction be stabilizing the transition state. They can also create new reaction pathways...
Khan Academy
Khan Academy: Reaction Rates: Introduction to Kinetics
A video lecture that focuses on kinetics, the study of how reactions progress and the rate of reactions. Kinetics, activation energy, activated complex and catalysts are discussed. [15:27]
Khan Academy
Khan Academy: Energy and Transport: Enzymes
Enzymes as catalysts for reactions in biological systems; discussion of substrates, active sites, induced fit, and activation energy. [8:12]
Khan Academy
Khan Academy: Catalysts
This video [7:30] explains how a catalyst speeds up a chemical reaction by lowering the activation energy.