Lawrence Berkeley National Laboratory
Berkeley Lab: The Atom
Presented is an overview of atomic theory concentrating on the experiments of Ernest Rutherford.
Simon Fraser University
Chem1 Virtual Textbook: Electron Spin and the Exclusion Principle
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses electron spin with related topics, such as the Pauli exclusion principle, quantum numbers, and more.
Simon Fraser University
Chem1 Virtual Textbook: Electronegativity
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses shared chemical bonds between two elements resulting in electronegative and electropositive outcomes.
Wikimedia
Wikipedia: Amorphous Solid
This page from the Wikipedia encyclopedia on amorphous solids contains information on examples of, the difference between amorphous solids and crystalline solid, and the transition between a very viscous liquid and an amorphous solid.
Museum of Science
The Atom's Family: The Phantom's Portrait Parlor
In this activity, students investigate the atomic world by attempting to reduce paper to the size of an atom. Although students won't be able to cut the paper that small, they will gain a better understanding of how small the atom is.
Michigan Reach Out
Guess What! A Lesson About Atoms
A lesson plan outlining a very simple way to introduce students to how the structure of the atom was determined. Uses a mystery box with surprise items inside.
Science for Kids
Science Kids: Science Quizzes: Atom Quiz
A ten-question true-or-false quiz on facts about the atom.
Thomas Jefferson National Accelerator Facility
Jefferson Lab: All About Atoms
The three basic particles that make up atoms are defined and illustrated.
School of Biological and Chemical Sciences, Queen Mary University of London
Queen Mary University: Glossary
This Queen Mary University site features a glossary of chemical terms, including London forces.
Other
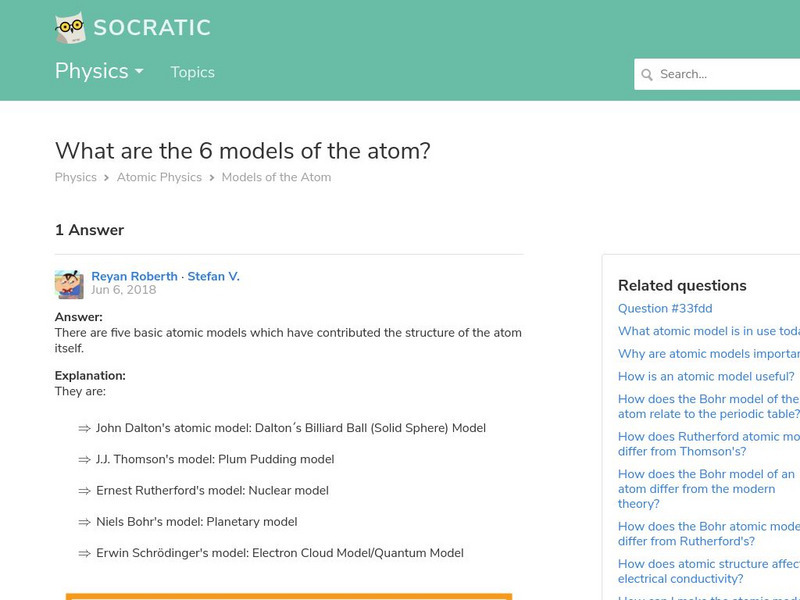
Socratic: What Are the 6 Models of the Atom?
Brief explanation of six major models of the atom along with illustrations. Covers the Greek model (Democritus), John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern electron cloud or quantum mechanics model.
Other
Atoms in Motion: All Matter Is Made of Atoms
Atoms are very, very small. Atoms are so small that it is often said that there are as many atoms in a single grain of sand as there are grains of sand on all of the world's beaches - certainly a difficult thing to prove, but you get the...
Other
Atoms in Motion: Atoms and Ions
All matter is made of atoms! And atoms are made of even smaller more fundamental particles. Atoms are not shiny little rigid spheres as we depict in drawings and simulations; they're more like a squishy storm of tiny particles swirling...
Other
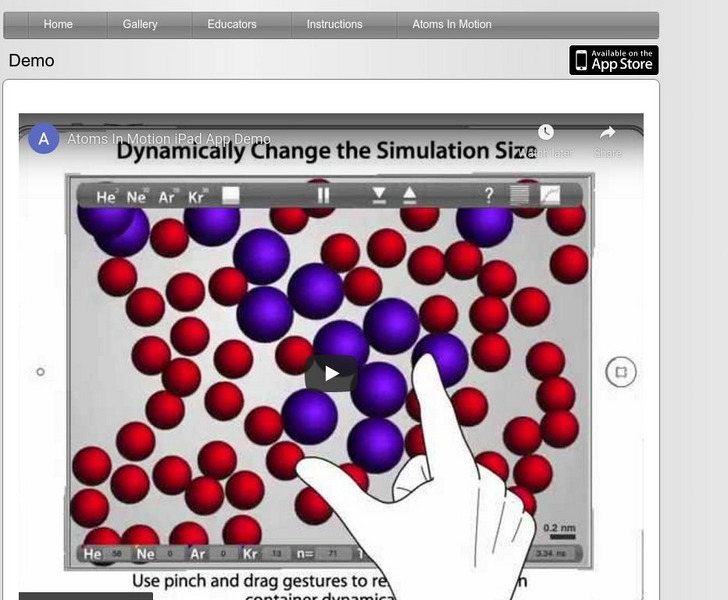
Atoms in Motion: Demo
Demonstration of the "Atoms In Motion" app running on an iPad simulator. User interactions and gestures control the entire simulation.
Other
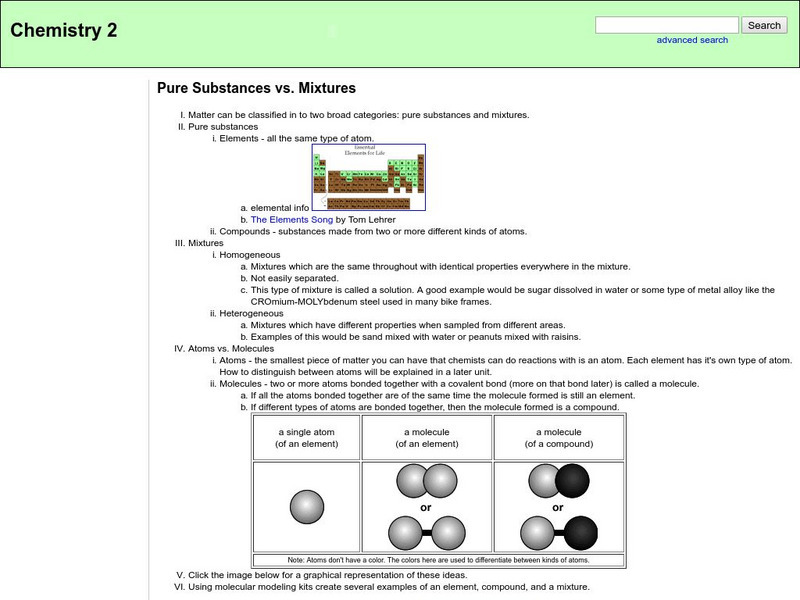
Chemsite: Pure Substances vs. Mixtures
An outline on the topics of pure substances and mixtures.
State University of New York
State University of New York: Paramagnetic and Diamagnetic Atoms
Make paramagnetic and diamagnetic atoms.
Able Media
On the Nature of the Universe: Study Guide
A study guide for Lucretius's work, "On the Nature of the Universe."
Clackamas Community College
Clackamas Community College: Combination Reactions
Synthesis reactions, also known as combination reactions, are explained in this chemistry tutorial. A few examples are shown. Links to practice examples and answers are provided.
Other
Popular Mechanics: Science
Popular Mechanics covers science and technology developments, including the latest trends, breakthroughs, background, analysis and information in science today. Updated every 20 minutes.
Sophia Learning
Sophia: Isotopes: Lesson 9
This lesson will define an isotope and explain what happens if the number of neutrons in an atom changes. It is 9 of 9 in the series titled "Isotopes."
Sophia Learning
Sophia: Isotopes: Lesson 1
This lesson will define an isotope and explain what happens if the number of neutrons in an atom changes. It is 1 of 9 in the series titled "Isotopes."
Sophia Learning
Sophia: Isotopes: Lesson 2
This lesson will define an isotope and demonstrate how to determine the average atomic mass of an element. It is 2 of 9 in the series titled "Isotopes."
Sophia Learning
Sophia: Isotopes: Lesson 8
This lesson will define an isotope and demonstrate how to determine the average atomic mass of an element. It is 8 of 9 in the series titled "Isotopes."
Sophia Learning
Sophia: Isotopes: Lesson 7
This lesson will introduce isotopes, and explain how they differ from other elements. It will also show that some isotopes are unstable and emit particles and/or radiation. It is 7 of 9 in the series titled "Isotopes."
Sophia Learning
Sophia: Models of the Atom: Lesson 2
Describe how the model of the atom advanced as scientific knowledge increased. This lesson is 2 of 3 in the series titled "Models of the Atom."