Hi, what do you want to do?
Chemistry Collective
Chem Collective: Solubility and the Common Ion Effect
Determine the concentration of an unknown NaCl solution using the common ion effect.
Chemistry Collective
Chem Collective: Metal Ligand Binding
Use silver chloride to determine the binding constant for a metal ligand complex.
Chemistry Collective
Chem Collective: Heats of Reaction: Hess's Law
This activity provides a demonstration of Hess's Law using three reactions: the solubility NaOH in water, the solubility NaOH in HCl, and the reaction of a solution of HCl and a solution of NaOH.
Chemistry Collective
Chem Collective: Temperature and the Solubility of Salts
Examine the solubilities of salts based on temperature.
Chemistry Collective
Chem Collective: Determining the Solubility Product
Determine the solubility product constatnt (Ksp) for various solids with this virtual lab.
Chemistry Collective
Chem Collective: Determining the Solubility of Copper Chloride
Given the solubility of CuCl at two different temperatures, predict its solubility at a third temperature. Then test your prediction by creating the solution in the virtual lab.
CK-12 Foundation
Ck 12: Factors Affecting Solubility
[Free Registration/Login may be required to access all resource tools.] Through readings, videos clips, and review questions, study the factors that affect solubility.
Concord Consortium
Concord Consortium: Stem Resources: Solubility
Learners can use this web-based activity to study solutions, as far as the intermolecular attractive forces that allow or do not allow a solvent to dissolve in a solute. Also discussed includes the solubility rule "like dissolves like"...
Frostburg State University
Why Does the Solubility of Gases Usually Increase as Temperature Goes Down?
This chemistry course article, "Why does the solubility of gases usually increase as temperature goes down?," provides text and related links on the effects of temperature and pressure on the solubility of gases.
American Chemical Society
Inquiry in Action: Solubility Test
A lab activity where students will identify an unknown crystal by applying a dissolving test.
American Chemical Society
Middle School Chemistry: Lesson Plans: Using Dissolving to Identify an Unknown
Students first observe a solubility test between salt and sugar. Next, they design their own solubility test with four known crystals and an unknown to discover the identity of the unknown.
PBS
Pbs Learning Media: Matter's Physical Properties: Interactive Lesson
In this interactive lesson, learn what the "physical properties" of matter are and how can they be measured and observed.
Savvas Learning
Chemistry: The Central Science/metathesis Reactions
Discussion of metathesis (double replacement) reactions.
Texas Instruments
Texas Instruments: Soluble or Not?
This StudyCard activity allows students to apply solubility rules to aqueous solutions. Students then determine whether the compounds are soluble or not in aqueous solution.
Science Education Resource Center at Carleton College
Serc: Mixtures, Solutions and Saturation: Testing Household Materials
Choosing two different powders, students will evaluate the properties of the materials and if the materials, when mixed with 50 ml of water, make a mixture or solution. They will examine the difference between a mixture and a solution...
Science Education Resource Center at Carleton College
Serc: Bubbling Blobs
For this chemistry lab, students investigate how oil and water don't mix. They will work on their observation skills and their ability to follow directions to ensure they get the correct results. Students can then develop a new, testable...
Estrella Mountain Community College
Online Biology Book: Chemistry Ii: Water and Organic Molecules
Online biology textbook discussing the chemical nature of water, and the importance of its molecular structure to life. Also discusses at length the organic molecules nucleic acids, proteins, lipids, and carbohydrates.
Purdue University
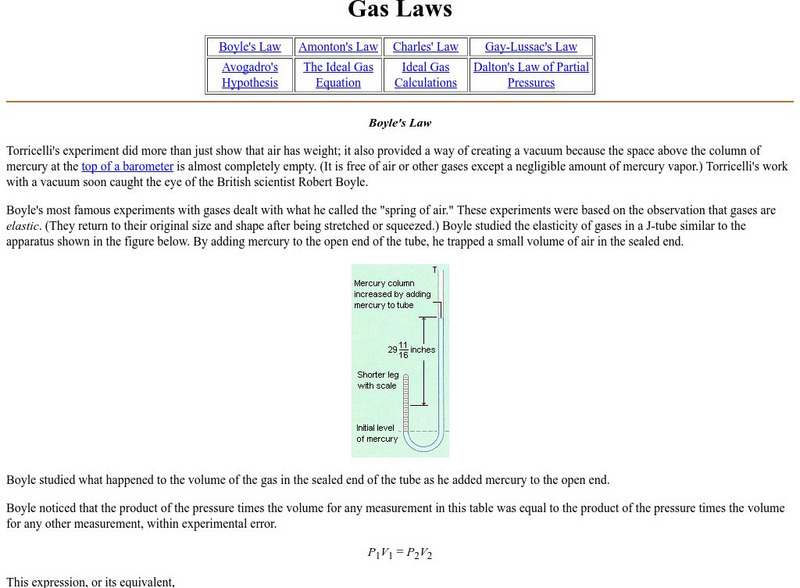
Gas Laws
This chemistry tutorial explains the gas laws (e.g., Boyle's Law) with applications.
Crescent Public Schools
The Internet Science Room: Solution Concentration
Students have the opportunity to study examples and worked out example problems to further their understanding of chemical solution concentration.
Other
Dr. Rod Beavon: The Solubility of Ionic Compounds
A detailed discussion of solubility as a function of Enthalpy and Entropy. Goes way beyond the standard solubility discussion.
State University of New York
State University of New York: The Common Ion Effect
This simulation explores the effect of secondary dissolved salts on the solubility of sparingly soluble salts.
State University of New York
State University of New York: Common Ion Effect in Acid Base Systems
This simulation uses solutions of weak acids and examines the response of this equilibrium system to the addition of the corresponding conjugate base.
Other
Elmhurst College: Virtual Chembook: What Are Physical Properties and Changes?
Brief descriptions of physical properties, physical changes, and the process of sublimation. The three states of matter, melting point, and boiling point are described. There is one link on the page, which leads to an explanation of...