Hi, what do you want to do?
CK-12 Foundation
Ck 12: Biology: Water and Life
[Free Registration/Login may be required to access all resource tools.] Covers the structure and properties of water.
CK-12 Foundation
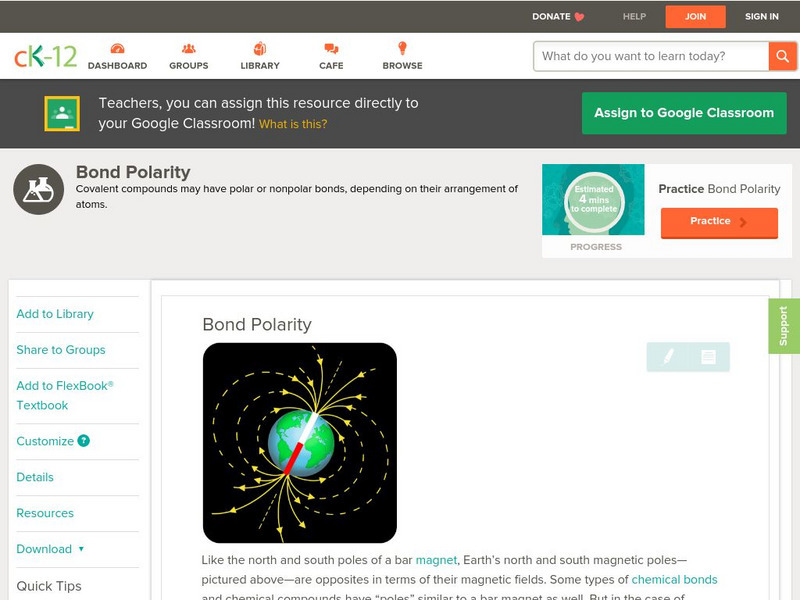
Ck 12: Chemistry: Bond Polarity
[Free Registration/Login may be required to access all resource tools.] Covers polarity and covalent bonds.
Lumen Learning
Lumen: Boundless: Magnetism and Magnetic Fields: Permanent Magnets
This short module covers permanent magnets, the concept of polarity, and the manufacture of permanent magnets. Includes some quiz questions.
Concord Consortium
Concord Consortium: Stem Resources: Electrons in Atoms and Molecules
A module with animations, explanations, and questions about the importance of electrons in the structure of an atom. Understand the definition and locations of electrons in the atom. Explore the role of electrons in bonding, polarity,...
Concord Consortium
Concord Consortium: Stem Resources: Intermolecular Attractions
Learn that boiling point, solubility, and DNA are affect by intermolecular forces in this module. Module includes lessons with questions and animations to explain London dispersion and dipole-dipole attractions. To conclude the lessons,...
Frostburg State University
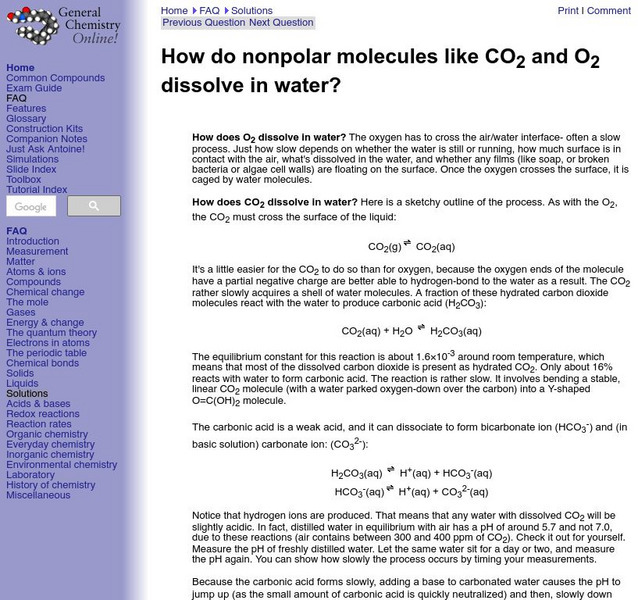
University of Frostburg: How Nonpolar Molecules Dissolve
This site from the University of Frostburg provides an explanation of the process by which nonpolar molecules dissolve in water.
CK-12 Foundation
Ck 12: Physical Science: Bond Polarity
[Free Registration/Login may be required to access all resource tools.] Polarity and covalent bonds.
Concord Consortium
Concord Consortium: Boiling Point of Polar and Nonpolar Substances
Control the temperature and observe the impact on the motion and interactions between particles. Compare the interactions between nonpolar molecules and between polar molecules to see how polarity affects the boiling point of substances...
Concord Consortium
Concord Consortium: What Makes Water Special?
Activity 2 investigates Why is water different from other liquids? This activity will investigate properties of different liquids. Students will explore what makes honey gooey and thicker than water. And why some liquids evaporate faster...
University of Georgia
University of Georgia: Polar Equations
This site discusses how to graph a polar equation and gives several pretty graphed examples.
McMaster University
Mc Master University: Molecular Structure
This PowerPoint presentation features 33 slides that explain chemical bonding and molecular shape.
San Diego State University
San Diego State University: Nonpolar Solute
A knowledge map with clickable links related to nonpolar solutes.
Other
Chemical Bonds: Electronegativity
A slideshow with several slides dedicated to electronegativity, the trend which is illustrated in the slide at this link. Others slides explain how the electronegativity can be used to determine bond character.
Sophia Learning
Sophia: Polarity of Molecular Compounds: Lesson 2
This lesson will demonstrate how to use molecular shape to determine if a molecule is polar or nonpolar. It is 2 of 2 in the series titled "Polarity of Molecular Compounds."
Educaplus (Jesús Peñas Cano)
Educaplus: Polaridad De Los Enlaces [In Spanish]
Test your knowledge by sorting the links from the lowest to greatest polarity.
McMaster University
Mc Master University: Electronegativity
This site is provided for by the McMaster University. Slides 34-36 in this presentation define electronegativity and relate it to bond polarity.
Frostburg State University
General Chemistry: Why Oh, Nh, and Fh Bonds Are Polar
Frostburg State University provides a brief explanation to the question of why small hydrogen molecules, such as OH, NH, and FH, are very polar.














![Educaplus: Polaridad De Los Enlaces [In Spanish] Unknown Type Educaplus: Polaridad De Los Enlaces [In Spanish] Unknown Type](https://content.lessonplanet.com/knovation/original/180051-fd27cf1353791aa3603432e82287e753.jpg?1661774243)

