Science ABC
Why Doesn't Aluminum Foil Get (Feel) HOT When Removed from the Oven?
The thermal mass of an object is its ability to store or absorb heat. Things that are considered ‘difficult’ to heat generally have a high thermal mass. Brick or concrete, for example, heat up only after they’re provided with a lot of...
Curated Video
GCSE Physics - Internal Energy and Specific Heat Capacity #28
This video covers: - What internal energy is - Relationship between kinetic energy, internal energy and temperature - What specific heat capacity is - How to use specific heat capacity in an equation General info: - Suitable for all GCSE...
Catalyst University
General Chemistry | Heat Capacity (q=smΔT) [Example 2]
Here, we perform a sample calculation for heat capacity in which we solve for a temperature (T) using q=smΔT.
Catalyst University
General Chemistry | Heat Capacity (q=smΔT) [Example 1]
Here, we perform a sample calculation for heat capacity in which we solve for heat (q) using q=smΔT.
Mazz Media
Specific Heat Capacity and Heat Capacity
This video defines the difference between heat capcity and specific heat capacity. In this program students will learn why different substances heat up and cool down at different rates and come to understand how specific heat capacity of...
Crash Course
Calorimetry
When the chemists who designed hand warmers were working, they had to consider how much heat they could give off to keep people warm — and not burn anyone in the process. How is this heat given off in a chemical reaction measured?...
DoodleScience
U-Values and Specific Heat Capacity
If you want to keep warm, learn how chemistry can help you choose materials when building a house. The video informs pupils how to interpret a u-value in relation to a given material. In addition, the instructor explains specific heat...
Khan Academy
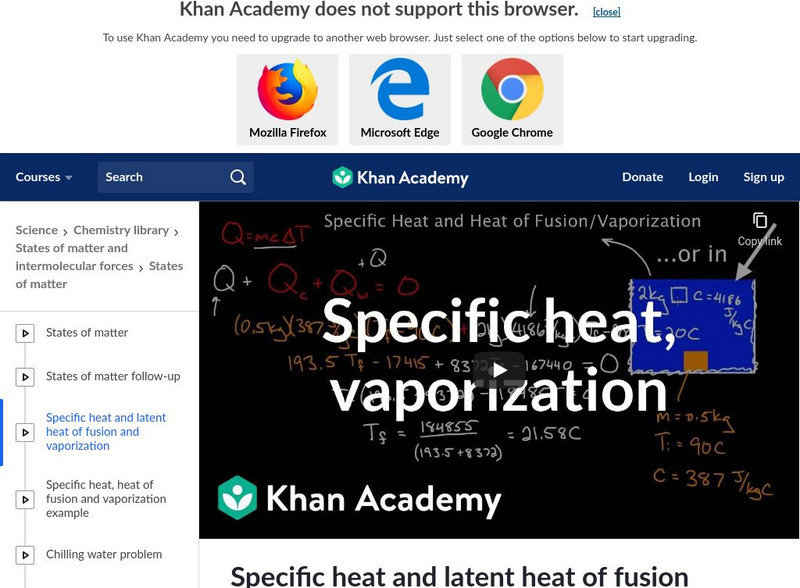
Khan Academy: Specific Heat and Latent Heat of Fusion and Vaporization
A video lecture explaining specific heat, heat of fusion, and the heat of vaporization. See a step-by-step example of how to calculate the amount of heat needed to change the temperature of water. Also explored is the energy required to...
Khan Academy
Khan Academy: Specific Heat and Latent Heat of Fusion and Vaporization
Defining specific heat, heat of fusion, and heat of vaporization, learn how to calculate the amount of heat to change the temperature of water and the energy required to change for a phase change. [14:57]
Khan Academy
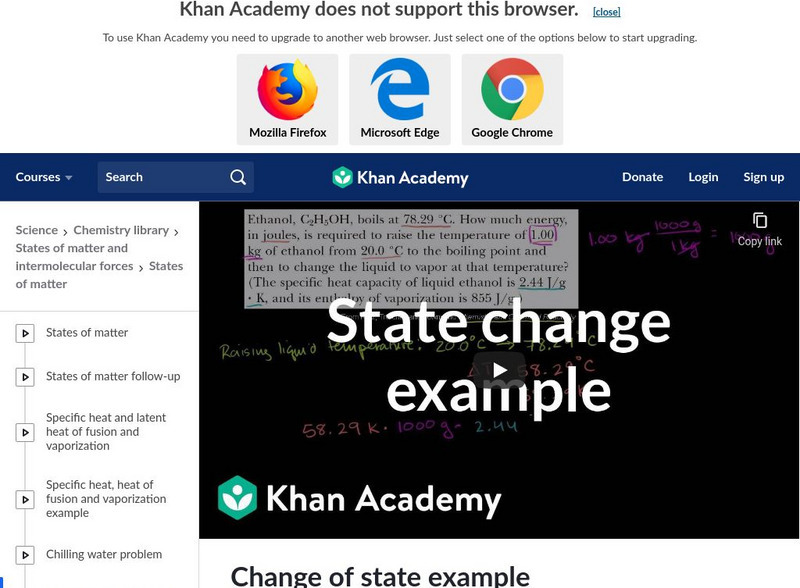
Khan Academy: Change of State Example
This is a specific heat capacity and enthalpy of vaporization example: calculating how much energy it takes to vaporize 1.00 kg of ethanol starting from 20 degrees C. [7:38]
Khan Academy
Khan Academy: Secific Heat and Latent Heat
Definitions of heat-related concepts. Calculations of heat to show changes in temperature and state of matter. [14:57]
Khan Academy
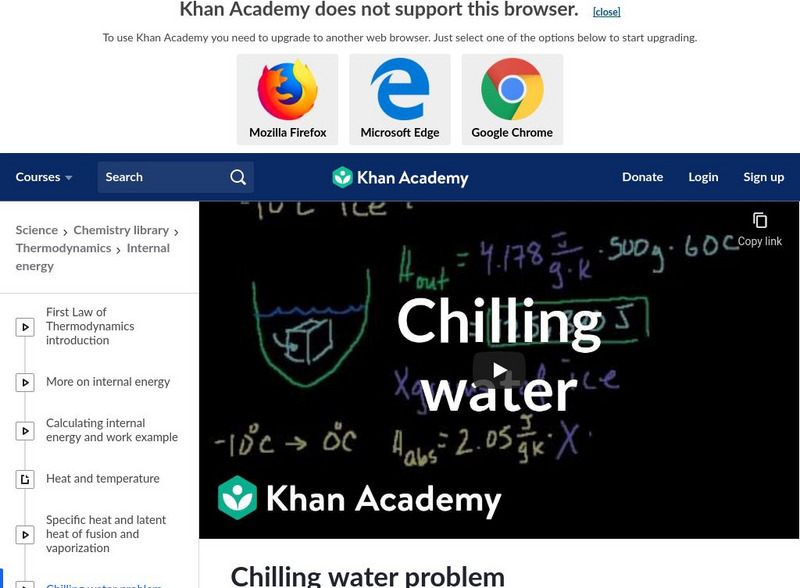
Khan Academy: Chilling Water Problem
An example of how ice can cause energy to transfer and reduce the temperature of water. [11:23]
Khan Academy
Khan Academy: Biology: Water, Acids, and Bases: Specific Heat of Water
Learn the basics specific heat in this video. Understand how water can control temperature. [10:49]
Khan Academy
Khan Academy: Chemistry: Change of State Example
A video lecture solving a specific heat and heat of vaporization problem found in a chemistry textbook. The video shows how to calculate the energy needed to raise the temperature and vaporize the liquid. [7:38]
University of Florida
Chemistry 2041 Lecture Notes: Phases and Equilibrium
An extensive set of notes and graphics (excellent graphics) pertaining to the phases of matter, phase changes and the energy changes associated with changes in phase. Characteristic properties of solids, liquids and gases are discussed....
Sophia Learning
Sophia: Define Specific Heat
This lesson will define specific heat of a substance and provide examples, using units of calories and joules.
Sophia Learning
Sophia: Energy Diagram of a Chemical Reaction
This lesson will introduce the energy diagram of a chemical reaction, and label the parts of the diagram.
Crash Course
Crash Course Chemistry #19: Calorimetry
This crash course episode dives into how enthalpy works. Learn how it is calculated and how it is determined experimentally. [11:57]
Khan Academy
Khan Academy: Chemistry: Chilling Water Problem
Figure out how many grams of ice is needed to lower the temperature of water by sixty degrees in this video lecture. The lecture explains how the ice absorbs heat from the water to lower the temperature. Using the specific heat of water...
Khan Academy
Khan Academy: Chemistry: Specific Heat, Heat of Fusion and Evaporation
The concept of specific heat capacity and the heat of fusion are both reviewed in this video. [14:49]
Khan Academy
Khan Academy: Specific Heat and Latent Heat of Fusion and Vaporization
Defining specific heat, heat of fusion, and heat of vaporization. How to calculate the amount of heat to change the temperature of water and the energy required to change for a phase change. [14:57]
Khan Academy
Khan Academy: Change of State Example
A video over viewing how to calculate how much energy it takes to vaporize 1.00 kg of ethanol starting from 20 degrees C. Learn about specific heat capacity and enthalpy of vaporization. [7:37]
Khan Academy
Khan Academy: Thermal Conduction
Intuition behind how heat gets transferred through thermal conduction. [7:15]
Khan Academy
Khan Academy: Intuition Behind Formula for Thermal Conductivity
Intuition behind formula for thermal conductivity. [6:19]