Hi, what do you want to do?
Other
Sir Joseph John Thomson
Have you ever wondered who discovered the electron? The answer is Nobel Prize winning physicist Sir Joseph John Thomson.
Other
Chemtopics: Development of Modern Atomic Theory [Pdf]
A summary of the achievements of J. J. Thomson, Ernest Rutherford, Niels Bohr, and Erwin Schrodinger.
Clackamas Community College
Clackamas Community College: Octete Rule
This resource offers a brief description of the Octet rule, along with a quick practice, and answers to the exercise.
Sophia Learning
Sophia: Jj Thomson: Lesson 1
This lesson explains JJ Thomson's experiments that led to the discovery of the electron. It is 1 of 3 in the series titled "JJ Thomson."
Sophia Learning
Sophia: Subatomic Particles: The Electron: Lesson 3
This lesson will explain that electrons are negatively charged particles with negligible mass and are found in pairs in orbitals surrounding the nucleus of an atom. It is 3 of 3 in the series titled "Subatomic Particles: The Electron."
Ducksters
Ducksters: Science for Kids: The Atom
Kids learn more about the science of the atom. Electrons, neutrons, and protons make up the smallest bits of matter.
Physics4kids
Physics 4 Kids: Where Traditional Physics Stops
We're about to move into the modern age of physics. In the early 1800's, scientists began examining the basis of matter, space, and time. Sometimes it gets very confusing, but the big idea is that Newton's physics describe about 90% of...
Sophia Learning
Sophia: Atomic Mass: Lesson 3
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. It is 3 of 4 in the series titled "Atomic Mass."
Sophia Learning
Sophia: Atomic Mass: Lesson 4
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. Module includes a slideshow and a quiz.
Sophia Learning
Sophia: Atomic Mass: Lesson 1
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. It is 1 of 4 in the series titled "Atomic Mass."
Sophia Learning
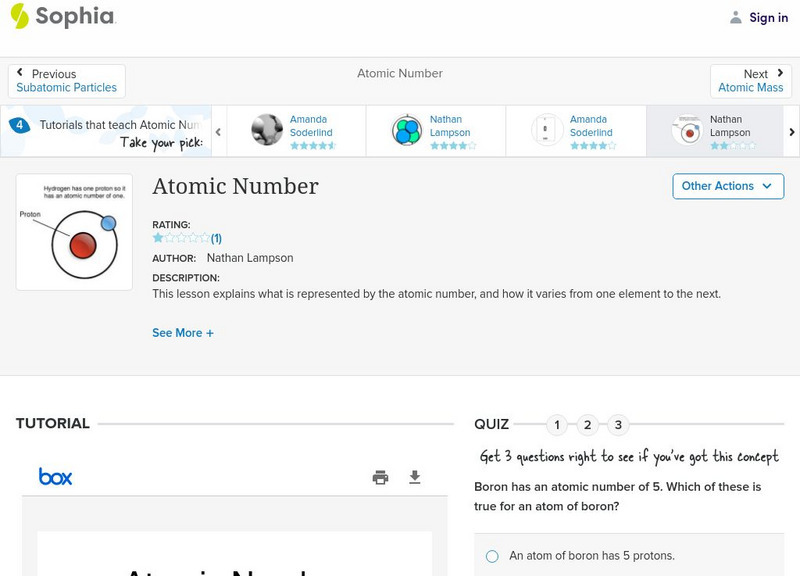
Sophia: Atomic Number: Lesson 3
This lesson explains what is represented by the atomic number, and how it varies from one element to the next. It is 3 of 7 in the series titled "Atomic Number."
Sophia Learning
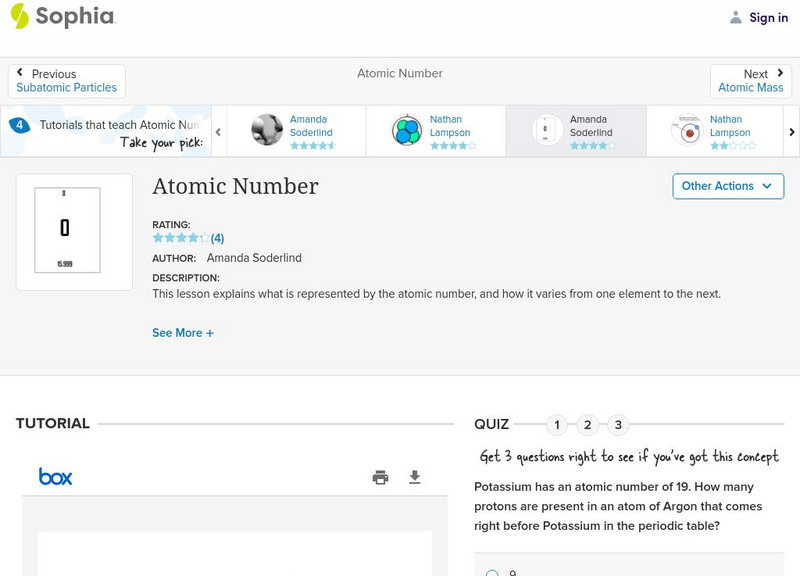
Sophia: Atomic Number: Lesson 6
This lesson explains what is represented by the atomic number, and how it varies from one element to the next. It is 6 of 7 in the series titled "Atomic Number."
Sophia Learning
Sophia: Characteristics of Chemical Bonds: Lesson 2
This lesson will present the basic properties and characteristics of chemical bonds. It is 2 of 4 in the series titled "Characteristics of Chemical Bonds."
Sophia Learning
Sophia: Characteristics of Chemical Bonds: Lesson 4
This lesson will present the basic properties and characteristics of chemical bonds. It is 4 of 4 in the series titled "Characteristics of Chemical Bonds."
Ducksters
Ducksters: Kids Science: Electricity 101
Kid's learn about the basic science of electricity. What is it and how it works.
Sophia Learning
Sophia: Atomic Mass: Lesson 2
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. It is 2 of 4 in the series titled "Atomic Mass."
Sophia Learning
Sophia: Atomic Number: Lesson 5
This lesson explains what is represented by the atomic number, and how it varies from one element to the next. It is 5 of 7 in the series titled "Atomic Number."
Physics Classroom
The Physics Classroom: Static Electricity Review
This review from the Glenbrook South High School provides a series of questions on various topics associated with static electricity (such as electrical insulation). Answers and explanations are hidden, yet easily accessed from within a...
ClassFlow
Class Flow: Element Math
[Free Registration/Login Required] In this flipchart students determine the number of protons, neutrons, and electrons of elements essential to life. They use Activotes to check knowledge gained. Students create a Bohr Model.
ClassFlow
Class Flow: Intro to Atoms
[Free Registration/Login Required] This flipchart was converted from Power Point and is used to introduce the history and concept of the Atom.
Simon Fraser University
Chem1 Virtual Textbook: Movement of the Electron
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site seeks to answer the question, "Why doesn't the electron fall into the nucleus?"
Tech Target
What Is: Cathode Ray Tube
This resource provides a good description of the cathode ray tube, with a very clear diagram and links to more in-depth information.
Science Struck
Science Struck: Electron Cloud Theory Explained
Explains the Uncertainty Principle, wave-particle duality, what the electron cloud model is, and how it came about as a result of the Schrodinger equation for Hydrogen.






![Chemtopics: Development of Modern Atomic Theory [Pdf] Handout Chemtopics: Development of Modern Atomic Theory [Pdf] Handout](https://static.lp.lexp.cloud/images/attachment_defaults/resource/large/FPO-knovation.png)