Science Education Resource Center at Carleton College
Serc: Coupled Plasma Mass Spectrometer to Teach About Water Chemistry
In this lesson students will use data acquired with an ICP-MS to teach them about water chemistry at a variety of levels.
TeachEngineering
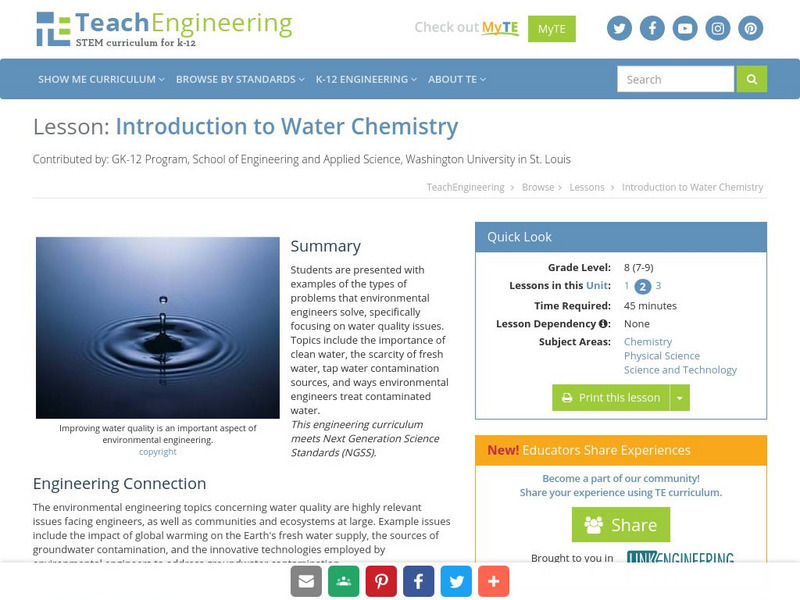
Teach Engineering: Introduction to Water Chemistry
Young scholars are presented with examples of the types of problems that environmental engineers solve, specifically focusing on water quality issues. Topics include the importance of clean water, the scarcity of fresh water, tap water...
American Chemical Society
Middle School Chemistry: Density of Water
Students discover the concept that density is a characteristic property of water by measuring the volume and mass of water and calculating its density.
American Chemical Society
Middle School Chemistry: Lesson Plans: Surface Tension
Students observe and investigate why water has a strong surface tension.
American Chemical Society
Middle School Chemistry: Changing State: Condensation
Students investigate water cycle processes by testing how cooling affects the rate of condensation of water vapor.
American Chemical Society
Middle School Chemistry: Finding Volume: Water Displacement Method
Students use the water-displacement method to find the volume of different rods that all have the same mass.
American Chemical Society
Middle School Chemistry: Chapter 3: Density
Six interactive chemistry lessons about density complete with handouts and animations.
American Chemical Society
Middle School Chemistry: Density: Sink and Float for Liquids
Students determine whether a liquid will sink or float in water by comparing its density to the density of water.
American Chemical Society
Middle School Chemistry: Density: Sink and Float for Solids
Students determine whether an object will sink or float by comparing its density to the density of water.
American Chemical Society
Middle School Chemistry: Lesson Plans: Water Is a Polar Molecule
Students develop their own water molecule model to help them understand the idea that water has a slight positive charge at one end of the molecule and a slight negative charge at the other.
American Chemical Society
Middle School Chemistry: Changing State: Freezing
Students investigate how low temperature causes water vapor to condense into a liquid and then freeze to form a solid.
National Geographic
National Geographic: Human Impacts on Marine Species
Students learn about three examples of human impacts on marine life: migration patterns and shipping, algal blooms and water chemistry, and marine debris. Some of these impacts are due to human activity in the ocean, and some impacts on...
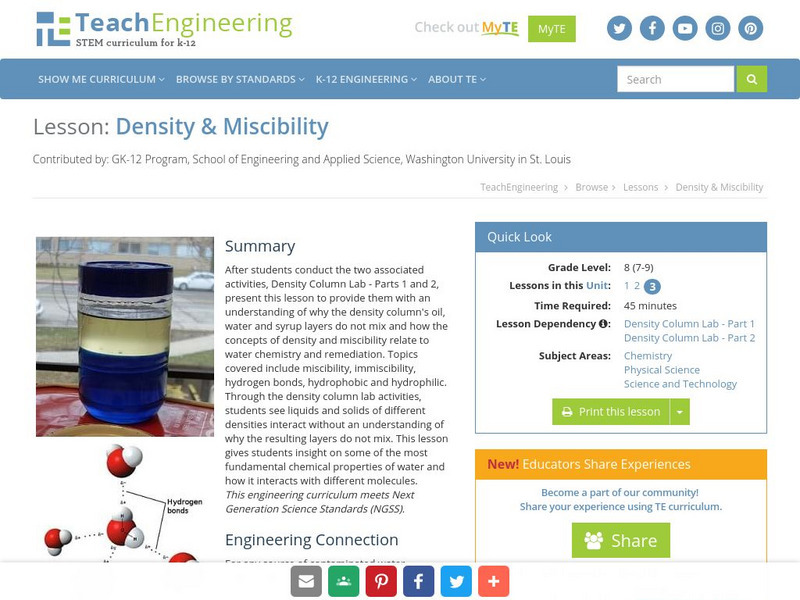
TeachEngineering
Teach Engineering: Density & Miscibility
After students conduct the two associated activities, Density Column Lab - Parts 1 and 2, present this lesson plan to provide them with an understanding of why the density column's oil, water and syrup layers do not mix and how the...
Other
Fnal: Supplying Our Water Needs
A cross-curricular lesson working out needs of a town where fish are dying in the local waters, how can water be purified, how daily activities impact the water supply, and what role does chemistry play in the way individuals use water...
American Chemical Society
Middle School Chemistry: Chapter 5: The Water Molecule and Dissolving
Nine exemplary chemistry lessons about the water molecule and dissolving complete with handouts and animations.
American Chemical Society
Middle School Chemistry: Lesson Plans: Why Does Water Dissolve Salt?
Students use their own model of a salt crystal and water molecule to show how water dissolves salt. Then, they relate their observations to the structure of salt, water, and alcohol on the molecular level.
American Chemical Society
Middle School Chemistry: Lesson Plans: Can Gases Dissolve in Water?
An activity through which young scholars learn why gas is able to dissolve in water, and how heat affects the solubility of a gas in a liquid.
American Chemical Society
Middle School Chemistry: Lesson Plans: Can Liquids Dissolve in Water?
Lesson plan with mulitmedia links in which students identify and control variables to help design a solubility test for different liquids in water.
American Chemical Society
Middle School Chemistry: Molecules Matter
Students observe and discuss water on the molecular level using the idea that water is composed of tiny molecules that are attracted to one another.
American Chemical Society
Middle School Chemistry: Changing State: Evaporation
Students build a model of a water molecule and design an experiment to see if adding energy affects the rate of evaporation.
American Chemical Society
Middle School Chemistry: Temperature and Density
Observe how heating and cooling affect the density of water. Combine the concepts of temperature, molecular motion, and density to learn that hot water is less dense than room temperature water and that cold water is more dense.
American Chemical Society
Middle School Chemistry: P H and Color Change
Students see a demonstration of a color change using universal pH indicator and then change the concentrations of an acid and a base using a universal indicator to test the pH of the resulting solutions. Through an animation, they see...
American Chemical Society
Middle School Chemistry: Carbon Dioxide Can Make a Solution Acidic
Students explain that carbon dioxide from any source reacts chemically with water to form carbonic acid. They will also be able to use the color changes of universal indicator to monitor the changing pH of a solution during a chemical...
American Chemical Society
Middle School Chemistry: Changing State: Melting
Discover the concept that energy transfer and molecular motion cause the change in state from a solid to a liquid. Also compare state changes of water to the state changes of other substances.
Other popular searches
- Lake Water Chemistry
- Water Chemistry Ions
- Co2 Water Chemistry
- Water Chemistry Lesson Plans
- Ocean Water Chemistry
- Basic Water Chemistry
- Water Chemistry Properties
- Storm Water Chemistry
- Marine Water Chemistry
- Size vs. Water Chemistry
- Chemistry of Water
- Water Chemistry Nitrate