American Chemical Society
Middle School Chemistry: Protons, Neutrons, and Electrons
Explore the particles that make up atoms: protons, electrons, and electrons.
American Chemical Society
Middle School Chemistry: Protons, Neutrons, and Electrons
Investigate why a charged object is attracted or repelled by another charged object. Explore the concept that the attraction between positive protons and negative electrons holds an atom together.
Science Struck
Science Struck: How to Find Protons, Neutrons and Electrons
Brief explanations of how to determine how many protons, neutrons, and electrons are in an element.
California State University
Csudh Project for Chemistry: Protons, Electrons, and Neutrons
This page is an exercise in relating the number of protons, electrons, and neutrons for an atom or monoatomic ion.
California State University
California State University: Proton, Electron, Neutron
An interesting and useful tool to practice recognition/calculation of atomic number, mass, and number of neutrons, electrons, etc. Can be used with all elements.
PBS
Nova: Atom Builder
Find out if you know enough about atoms to build them. The goal of the activity is to build an atom of a particular element by dragging the correct numbers of neutrons, protons and electrons into the atom.
Math Science Nucleus
Math/science Nucleus: Electrons and the Hairy Monster
This animation discusses electrons and the properties of electrons in a storybook format featuring hairy monsters, strange rocks, and fun animations.
Scholastic
Scholastic: Study Jams! Science: Matter: Atoms: Protons, Neutrons, Electrons
A video and a short quiz on the parts of an atom, the periodic table, and molecules.
Other
University of Kansas: Quarked!: Matter Mechanic
Build elements and molecules using neutrons, protons, and electrons. Choices include helium, carbon, oxygen, aluminum, water, and salt.
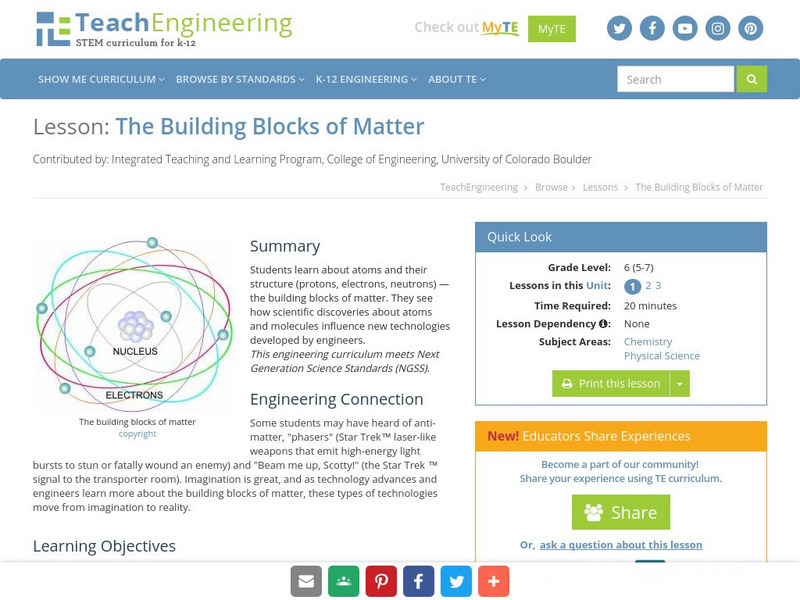
TeachEngineering
Teach Engineering: The Fundamental Building Blocks of Matter
This lesson plan explores the fundamentals of atoms and their structure. The building blocks of matter (protons, electrons, neutrons) are covered in detail. Students think about how atoms and molecules can influence new technologies...
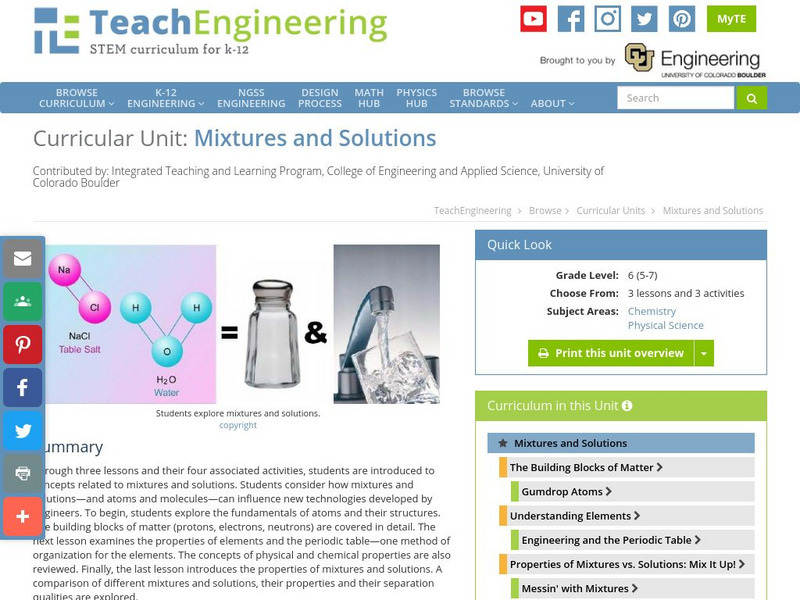
TeachEngineering
Teach Engineering: Mixtures and Solutions
This unit covers introductory concepts of mixtures and solutions. Students think about how mixtures and solutions, and atoms and molecules can influence new technologies developed by engineers. The first lesson explores the fundamentals...
Thomas Jefferson National Accelerator Facility
Jefferson Lab: It's Elemental: Element Math Game
The interactive activity examines the Periodic Table of Elements. Learners answer questions about the number of protons, electrons, neutrons, or nucleons that an atom of an element contains.
Concord Consortium
Concord Consortium: Atom and Ion Builder
Explore how changing the numbers of protons, neutrons, and electrons affect the type of atom.
Sophia Learning
Sophia: Subatomic Particles: The Electron: Lesson 3
This lesson will explain that electrons are negatively charged particles with negligible mass and are found in pairs in orbitals surrounding the nucleus of an atom. It is 3 of 3 in the series titled "Subatomic Particles: The Electron."
Other
Nuclear Twin: The Discovery of the Proton and Neutron
Trace the history of the discovery of protons and neutrons in this informative site.
Utah Education Network
Uen: Atom in a Bag
Students will use bags of beads with known quantities of electrons, neutrons and protons to identify the element that they represent.
CK-12 Foundation
Ck 12: Plix: Build Some Helium: Atoms to Molecules
[Free Registration/Login Required] Build your own helium atom and make sure it has the correct number of protons, electrons, and neutrons on this site. Site also includes a small quiz on the topic.
Tom Richey
Slide Share: Atomic Structure
Slideshow looking at the history of models of the atom, including those proposed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Discusses subatomic particles, including the numbers of protons, neutrons,...
Sophia Learning
Sophia: Subatomic Particles: The Neutron: Lesson 2
This lesson will explain that neutrons are particles in the nucleus that have no charge and a mass of one amu. It is 2 of 3 in the series titled "Subatomic Particles: The Neutron."
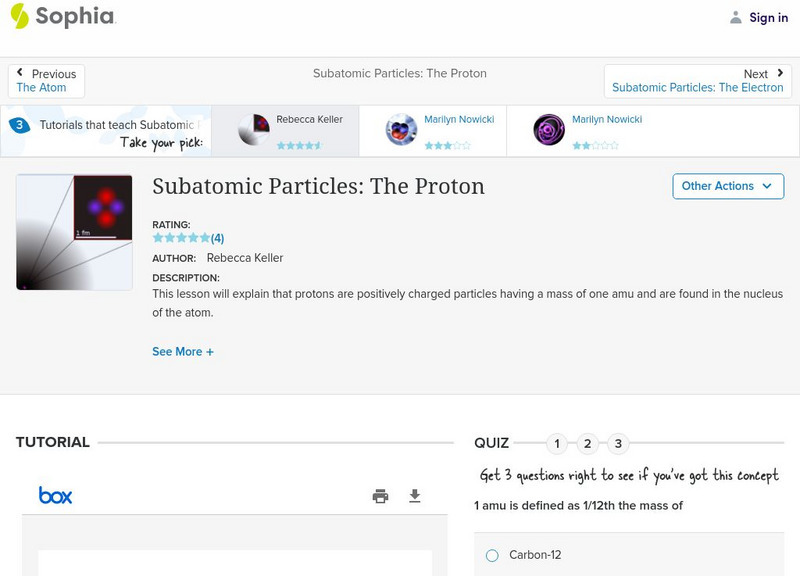
Sophia Learning
Sophia: Subatomic Particles: The Proton: Lesson 2
This lesson will explain that protons are positively charged particles having a mass of one amu and are found in the nucleus of the atom. It is 2 of 3 in the series titled "Subatomic Particles: The Proton."
ClassFlow
Class Flow: Element Math
[Free Registration/Login Required] In this flipchart students determine the number of protons, neutrons, and electrons of elements essential to life. They use Activotes to check knowledge gained. Students create a Bohr Model.
Mocomi & Anibrain Digital Technologies
Mocomi: Structure of an Atom
An atom is made of three parts - protons, neutrons, and electrons. Here you can explore these different parts.
Estrella Mountain Community College
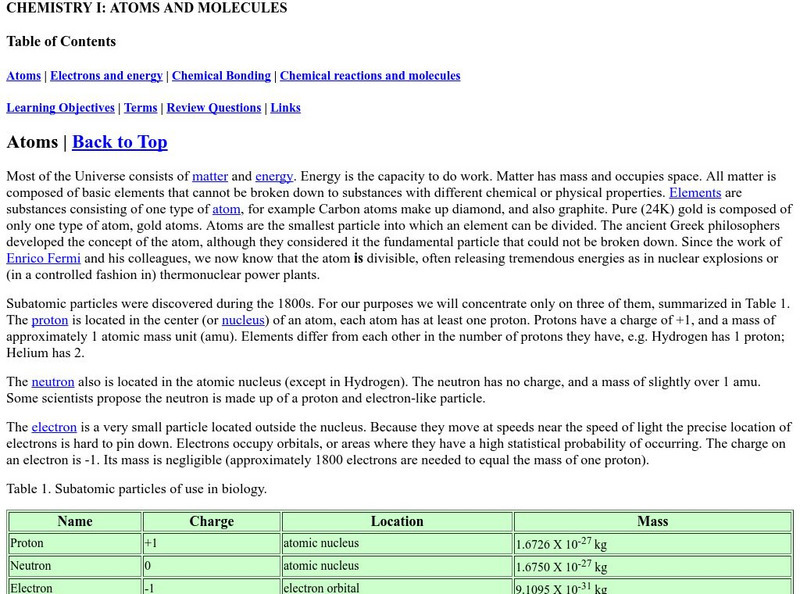
Online Biology Book: Chemistry I: Atoms and Molecules
In this online biology textbook, learn about atoms and molecules as they relate to life. Find out about topics such as electrons and energy, chemical bonding, and chemical reactions.
Lawrence Berkeley National Laboratory
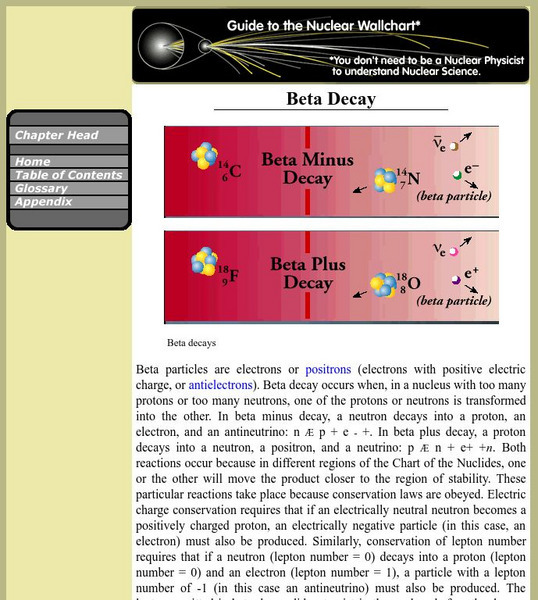
Berkeley Lab: Beta Decay
Entry explores the process of beta decay which occurs when, in a nucleus with too many protons or too many neutrons, one of the protons or neutrons is transformed into the other.