Other
Aquaholic: Charles' Law
Charles Law is defined and explained. Sample problems are given and solved. Links to related topics.
Science Education Resource Center at Carleton College
Serc: Charles' Law and Ivory Soap
With this activity students will observe and record the properties of various types of bar soap. They will discover that due to the ingredients some soaps are denser than others, and ivory soap will actually float. Students will see a...
CK-12 Foundation
Ck 12: Physical Science: Charles' Law
[Free Registration/Login may be required to access all resource tools.] Charles' law and the relationship between the volume and temperature of a gas.
CK-12 Foundation
Ck 12: Physical Science: Charles' Law
[Free Registration/Login may be required to access all resource tools.] Charles' law and the relationship between the volume and temperature of a gas.
TED Talks
Ted: Ted Ed: The Abc's of Gas: Avogadro, Boyle, Charles
In this video, Brian Bennett defines Boyle's Law, Charles' Law, and Avogadro's Law to help describe the properties of gas. [2:50] Followed by a short quiz and a list of additional resources to explore.
Mocomi & Anibrain Digital Technologies
Mocomi: What Is Charles' Law?
Definition, formula, and fun facts for Charles' Law.
Science Buddies
Science Buddies: Charles's Law
This is a modern version of a classic experiment by Jacques Charles on the volume of a gas at different temperatures. Charles discovered the relationship between volume and temperature of gases that now bears his name. This project shows...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Charles' Law: Volume Temperature Relationship
Colorful animation outlining the relationship between the temperature and volume of gas particles. See what happens when the number of particles and pressure remains constant and the temperature of the gas is altered.
Simon Fraser University
Chem1 Virtual Textbook: Charles' Law
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with gas laws and specifically with Charles' Law and Gay-Lussac's law.
Georgia State University
Georgia State University: Hyper Physics: Ideal Gas Law With Constraints
Charles' law is derived from the ideal gas law. An equation of the ideal gas law under pressure constraints is given and the derivation is explained.
Wikimedia
Wikipedia: Gas Laws
Wikipedia offers the definition of the gas laws, including Boyle's Law, Charles' Law, and Graham's Law. Also defines the term, "Ideal gas."
California State University
California State University: Charles' Law Problem Generator
Create an unlimited number of Charles' Law problems to practice. Generator reveals correct answer after three incorrect tries.
Georgia Department of Education
Ga Virtual Learning: Chemistry: Gas Laws
Through informational text, interactive practice problems, virtual simulations, and video clips, students learn about the gas laws.
Wikimedia
Wikipedia: Law of Charles and Gay Lussac
Wikipedia provides information on the Law of Charles and Gay-Lussac, a law that relates the volume and temperature of an ideal gas held at a constant pressure.
Simon Fraser University
Chem1 Virtual Textbook: The Basic Gas Laws
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals specifically with a series of gas laws including Boyle's law, Charles and...
CK-12 Foundation
Ck 12: Plix Series: Charles' Law: Gas Balloon
[Free Registration/Login Required] Observe what happens to the volume of a balloon with a change in temperature. After the activity, answer a multiple-choice, challenge question.
Science Education Resource Center at Carleton College
Serc: Mini Lab: Investigating Gas Laws
In this activity, young scholars investigate two gas laws: Charles and Boyle's. They will determine the relationship between gases and certain variables (temperature, volume and pressure), then watch a demonstration and determine what...
Crescent Public Schools
The Internet Science Room: The Gas Laws
A chemistry tutorial which explains the Gas Laws, and provides examples for further understanding.
Purdue University
Gas Laws
This chemistry tutorial explains the gas laws (e.g., Boyle's Law) with applications.
Wolfram Research
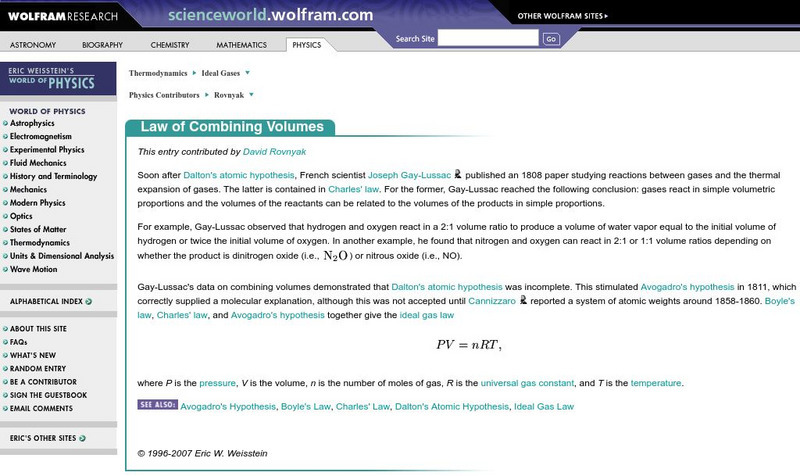
Wolfram Science World: Law of Combining Volumes
This site contains informaiton on the law of combining volumes. A formula is given and links to key terms.
University of Sydney (Australia)
Thermal Physics Module: Ideal Gases [Pdf]
A molecular model of a gas is introduced and explained. Assumptions behind the ideal gas law are presented. The ideal gas law is stated. Charles' law and Boyle's law are derived from the ideal gas law.
Wikimedia
Wikipedia: Boyle's Law
Wikipedia provides information on Boyle's Law, a gas law that relates the volume and pressure of an ideal gas held at a constant temperature.
Other
University California at Irvine: Introduction to Gas Laws
A set of exercises on the basics of gas laws. Includes links to several animations, although a few links are broken. Ends with a five-question quiz on gas laws.
TeachEngineering
Teach Engineering: Fluid Power Basics
Middle schoolers learn about the basic fundamental concepts regarding fluid power, which includes both pneumatic, which utilize gas, and hydraulic, which utilize liquid, systems. Both systems contain four basic components: a reservoir, a...