American Chemical Society
Change in Temperature - Exothermic Reaction
Alone, or as part of the intended unit on chemical reactions, this activity allows learners to experience an exothermic reaction. Here, learners add calcium chloride to a baking soda solution and watch the temperature rise! They will...
American Chemical Society
Production of a Gas - Controlling a Chemical Reaction
Though the publisher designated this unit for use with third through eighth grades, this particular lesson would be best used with middle schoolers due to the specific measurement skills required. Basically, they set up the reaction...
Chymist
Esters: An Introduction to Organic Chemistry Reactions
Scratch and sniff an introduction to organic chemical reactions. A creative lesson has individuals study the esters commonly used in scratch-and-sniff stickers and advertisements. Following the lab procedure, scholars create the organic...
Scholastic
Study Jams! Newton's Third Law: Action & Reaction
What happens when two teens collide? Find out when RJ runs into Chloe in the school hallway! After their collision, they discuss Newton's third law of motion. Their collision is replayed a few times to identify the action and reaction of...
Curated OER
Chemical Equations and Reactions
Graphic organizers, photos, diagrams, and text bring the world of chemical reactions to life. By viewing this presentation, young chemists learn how to recognize when a chemical reaction has occurred, and how to balance chemical...
American Chemical Society
Change in Temperature - Endothermic Reaction
Now that learners have been exposed to chemical changes, they learn that some take in heat and therefore, decrease in temperature. The same reaction that they have been investigating between baking soda and vinegar is revisited,...
Royal Society of Chemistry
A Solid-Solid Reaction between Lead Nitrate and Potassium Iodide
Why is it so difficult to make two solid compounds react? Investigate the concepts of particle collisions and rate of reaction using a quick demonstration. The colorful experiment features two plain, white solids combining to form a...
Curated OER
Observing Chemical Reactions
Start this series of lessons with a bang! Five exothermic reactions are outlined in this resource, including a demonstration that produces both light and sound. In the lab, chemistry apprentices record temperature changes, make hand...
Curated OER
Identifying and Explaining Reactions
Introduce high schoolers to chemical reactions with this series of activities. In a little over an hour, scientists observe four gas-producing reactions: the combination of hydrochloric acid and sodium hydroxide, placing pasta in...
Curated OER
Heat Energy Released or Absorbed in Chemical Reactions
Chemistry whizzes test the change in water temperature produced by a burning candle and the change in acid temperature produced by magnesium metal. With these two laboratory activities they explore heat produced during chemical...
Concord Consortium
Temperature and Reaction Rate
Does increasing temperature increase the rate of a chemical reaction? Junior chemists examine the effects of temperature on reaction rate using an engaging interactive. Pupils select the temperature of the reaction vessel, then observe...
Concord Consortium
What Is a Chemical Reaction?
Take your class inside a beaker for an up-close view of a chemical reaction! Junior chemists examine how chemical reactions occur using an interactive resource. The activity allows users to change the temperature and observe how it...
Concord Consortium
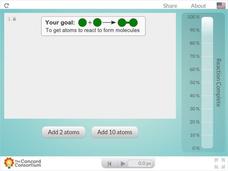
Concentration and Reaction Rate
Does concentration affect the rate of a chemical reaction? Science scholars observe and control a chemical reaction in an interactive simulation. The resource allows pupils to add atoms to the reaction vessel and monitor the reaction's...
Royal Society of Chemistry
Some Reactions of Carbon Dioxide—Microscale Chemistry
Precipitation reactions are always interesting. How about one that forms a precipitate using a gas? Chemists of any age will enjoy this twist on a standard solubility lab. Partners observe the lack of interaction between sodium hydroxide...
Concord Consortium
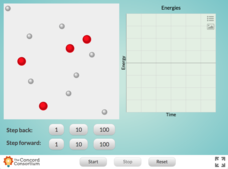
Chain Reaction Between Hydrogen and Oxygen
Looking for a simple way to teach conservation of energy in chemical reactions? Pupils can observe energy changes as water forms during a chain reaction between oxygen and hydrogen using an interactive. The resource instructs users to...
Science Geek
Reaction Types
This is one way to get a reaction from your classes! The lesson presents the different reaction types with an explanation, chemical equation model, and examples. The slides include decomposition, single replacement, double replacement,...
University of Georgia
Endothermic and Exothermic Reactions
Equip your chemistry class with the tools to properly understand endothermic and exothermic reactions. Young chemists collect, analyze, and graph data to determine how the Law of Conservation of Matter is applied to chemical composition...
American Chemical Society
Temperature and the Rate of a Chemical Reaction
Putting glow sticks in the freezer makes them last longer, but why is that? Lesson focuses on how temperature impacts the rate of a chemical reaction. It begins with a teacher demonstration, then scholars design their own experiments...
K20 LEARN
Untwining And Intertwining: Chemical Reactions
What happened when the chemistry teacher told a bad joke? There was no reaction! A creative take on the traditional reaction types lesson invites learners to draw their own conclusions about how compounds and elements combine. Groups...
Pingry School
Heat of Reaction and Hess's Law
Melting and burning might seem like opposites, but both exist as common examples of exothermic reactions. Scholars work with three different exothermic chemical reactions to determine the enthalpy changes. They measure and mix chemicals,...
Concord Consortium
Reaction Between Hydrogen and Oxygen Atoms
Is this resource a great way for your class to observe bonding between oxygen and hydrogen? OH yeah! Scholars learn about the changes in kinetic and potential energy as molecules of oxygen and hydrogen interact. Kinetic, potential, and...
Beyond Benign
A Green(er) Redox Reaction
Do some experimentation with reduction-oxidation! Stoichiometry superstars use a single-replacement reaction to study limiting reactant, theoretical yield, and the reactivity of metals through a lab activity. The teacher's guide includes...
Cornell University
Predicting Chemical Reactions
Prove the Law of Conservation of Mass through a lab investigation. A well-designed lesson asks groups to combine materials and monitor indicators for chemical reactions. Measuring the mass of the reactants and products allows individuals...
Cornell University
Chemical Reactions
Investigate the Law of Conservation of Mass through a lab exploration. Individuals combine materials to initiate chemical reactions. They monitor for signs of reactions and measure the masses before and after the reactions for comparison.
Other popular searches
- Chemical Reactions
- Chemistry Chemical Reactions
- Types of Chemical Reactions
- Endothermic Reactions
- Combustion Reactions
- Balance Chemical Reactions
- Chemical Reactions of Matter
- Single Replacement Reactions
- Precipitation Reactions
- Balancing Chemical Reactions
- Displacement Reactions
- Nuclear Reactions